QUESTION 16–1
To keep their action local, paracrine signal molecules must be prevented from straying too far from their points of origin. Suggest different ways by which this could be accomplished. Explain your answers.
Information can come in a variety of forms, and communication frequently involves converting the signals that carry that information from one form to another. When you receive a call on your mobile phone, for instance, the phone converts radio signals, which travel through the air, into sound waves, which you hear. This process of conversion is called signal transduction (Figure 16–2).
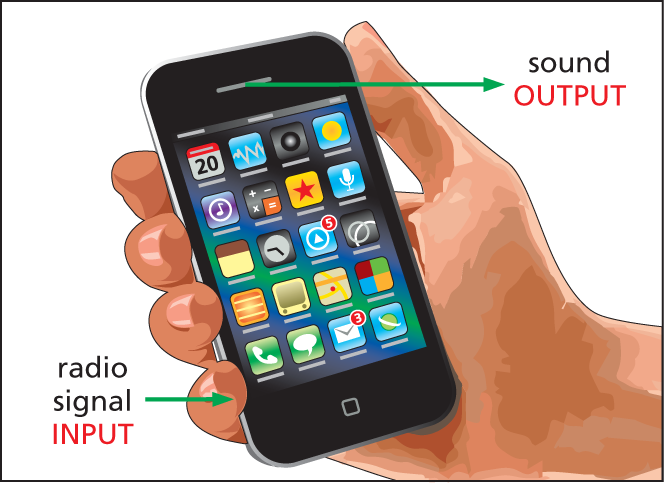
Illustration A shows a smartphone in a hand. An arrow pointing to the bottom left of the phone reads radio signal input. Another arrow pointing to the the top right from the phone reads sound output.
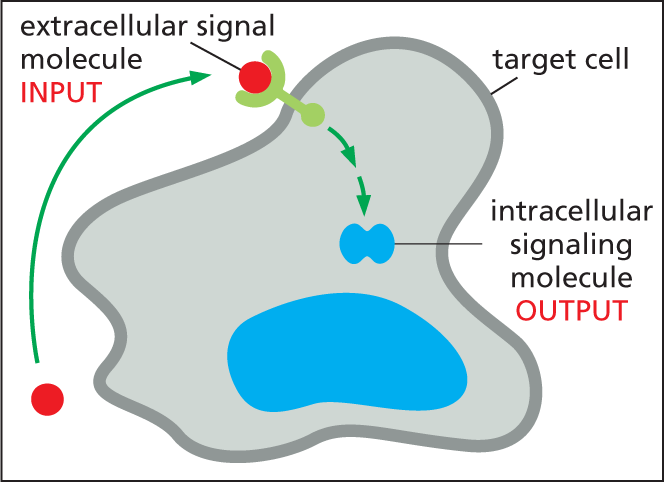
Illustration B shows an extracellular signal molecule entering an irregularly shaped target cell with a label saying input. Inside the cell, the former extracellular signal molecule is labeled as a intracellular signaling molecule, labeled output.
When cells communicate, a signaling cell will produce a particular type of extracellular signal molecule that is subsequently detected by a target cell. As in human conversation, most animal cells both send and receive signals, and they can therefore act as both signaling cells and target cells.
Target cells possess proteins called receptors that recognize and respond to specific signal molecules. Signal transduction begins when a receptor on the target cell receives an incoming extracellular signal and then produces intracellular signaling molecules that alter cell behavior. Most of this chapter is concerned with signal reception and transduction—the events that cell biologists have in mind when they refer to cell signaling. First, however, we look briefly at a few of the different types of extracellular signals that cells send to one another—and what happens when target cells receive those signals.
Cells in multicellular organisms use hundreds of kinds of extracellular signal molecules to communicate with one another. These signal molecules can be proteins, peptides, amino acids, nucleotides, steroids, fatty acid derivatives, or even dissolved gases—but they all rely on just a handful of basic styles of communication for getting the message across.
In multicellular organisms, the most “public” style of cell–cell communication involves broadcasting the signal throughout the whole body by secreting it into an animal’s bloodstream or a plant’s sap. Extracellular signal molecules used in this way are called hormones, and, in animals, the cells that produce hormones are called endocrine cells (Figure 16–3A). Part of the pancreas, for example, is an endocrine gland that produces several hormones—including insulin, which regulates glucose uptake in cells all over the body.
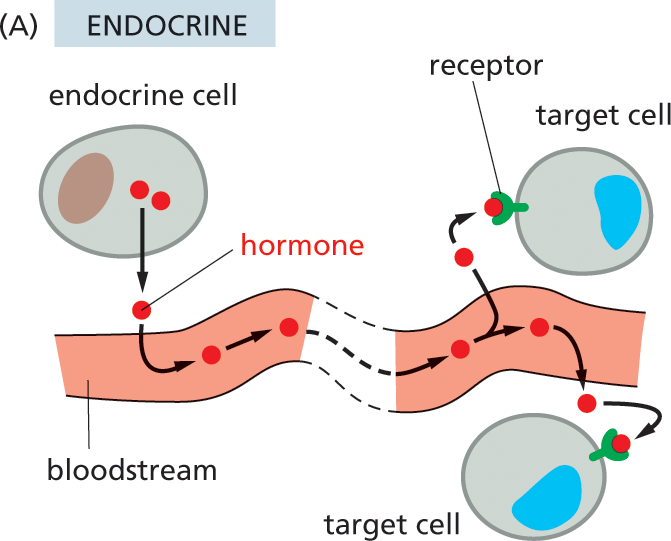
Illustration A shows how animal cells communicate with each other via the endocrine system. A blood vessel is shown, with hormones from an endocrine cell entering the bloodstream. The hormones travel through the bloodstream, exit the blood vessel, and bind to the receptors on the outside of two target cells.
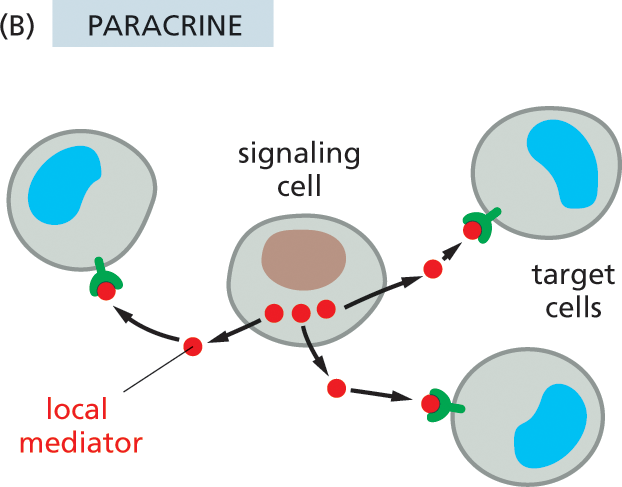
Illustration B shows how animal cells communicate with each other in the paracrine system. A signaling cell at the center of the image is surrounded by three target cells. The hormones from the signaling cell are transferred to the receptors on the target cells via local mediators.
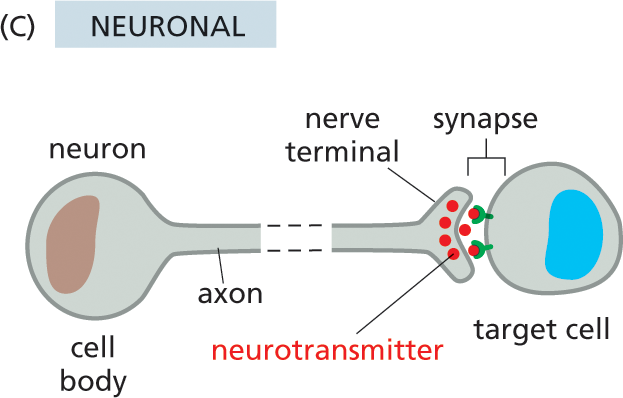
Illustration C shows neuronal cell signaling. A neuron and a target cell are shown. The small gap between the nerve terminal of the neuron and the target cell is labeled synapse. The cell body and the narrow axon of the neuron are labeled. Several small spheres labeled neurotransmitters are transferred from the nerve terminal to the receptors on the periphery of the target cell, across the synapse.
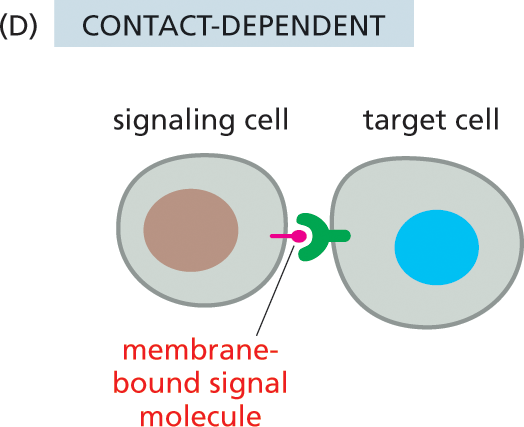
Illustration D shows contact-dependent signaling. A signaling cell and a target cell are shown. Attached to the signaling cell is a membrane bound signal molecule. The signal molecule is inside the receptor on the periphery of the target cell.
Somewhat less public is the process known as paracrine signaling. In this case, rather than entering the bloodstream, the signal molecules diffuse locally through the extracellular fluid, remaining in the neighborhood of the cell that secretes them. Thus, they act as local mediators on nearby cells (Figure 16–3B). Many of the signal molecules that regulate inflammation at the site of an infection or that control cell proliferation in a healing wound function in this way. In some cases, cells can respond to the local mediators that they themselves produce, a form of paracrine communication called autocrine signaling; cancer cells sometimes promote their own survival and proliferation in this way.
Neuronal signaling is a third form of cell communication. Like endocrine cells, nerve cells (neurons) can deliver messages over long distances. In the case of neuronal signaling, however, a message is not broadcast widely but is instead delivered quickly and specifically to individual target cells through private lines. As described in Chapter 12, the axon of a neuron terminates at specialized junctions (synapses) on target cells that can lie far from the neuronal cell body (Figure 16–3C). The axons that extend from the spinal cord to the big toe in an adult human, for example, can be more than a meter in length. When activated by signals from the environment or from other nerve cells, a neuron sends electrical impulses racing along its axon at speeds of up to 100 meters per second. On reaching the axon terminal, these electrical signals are converted into a chemical form: each electrical impulse stimulates the nerve terminal to release a pulse of an extracellular signal molecule called a neurotransmitter. The neurotransmitter then diffuses across the narrow (<100 nm) gap that separates the membrane of the axon terminal from that of the target cell, reaching its destination in less than 1 millisecond.
A fourth style of signal-mediated cell–cell communication—the most intimate and short-range of all—does not require the release of a secreted molecule. Instead, the cells make direct physical contact through signal molecules lodged in the plasma membrane of the signaling cell and receptor proteins embedded in the plasma membrane of the target cell (Figure 16–3D). During embryonic development, for example, such contact-dependent signaling allows adjacent cells that are initially similar to become specialized to form different cell types, as we discuss later in this chapter.
To get a better feel for these different signaling styles, imagine trying to publicize a potentially stimulating lecture—or a concert or sporting event. An endocrine signal would be akin to broadcasting the information over the radio. A more localized paracrine signal would be the equivalent of posting a flyer on selected notice boards near the arena—with an autocrine signal being a reminder you add to your own personal calendar. Neuronal signals—long-distance but personal—would be similar to a phone call, text message, or e-mail, and contact-dependent signaling would be like a face-to-face conversation.
Table 16–1 lists some examples of hormones, local mediators, neurotransmitters, and contact-dependent signal molecules. The actions of several of these are discussed in greater detail throughout this chapter.
|
TABLE 16–1 SOME EXAMPLES OF SIGNAL MOLECULES |
|||
|
Signal Molecule |
Site of Origin |
Chemical Nature |
Some Actions |
|
Hormones |
|||
|
Epinephrine (adrenaline) |
Adrenal gland |
Derivative of the amino acid tyrosine |
Increases blood pressure, heart rate, and metabolism |
|
Cortisol |
Adrenal gland |
Steroid (derivative of cholesterol) |
Affects metabolism of proteins, carbohydrates, and lipids in most tissues |
|
Estradiol |
Ovary |
Steroid (derivative of cholesterol) |
Induces and maintains secondary female sexual characteristics |
|
Insulin |
β cells of pancreas |
Protein |
Stimulates glucose uptake, protein synthesis, and lipid synthesis in various cell types |
|
Testosterone |
Testis |
Steroid (derivative of cholesterol) |
Induces and maintains secondary male sexual characteristics |
|
Thyroid hormone (thyroxine) |
Thyroid gland |
Derivative of the amino acid tyrosine |
Stimulates metabolism in many cell types |
|
Ethylene |
Plant tissues |
Gaseous hydrocarbon |
Regulates developmental processes, including seed germination and fruit ripening |
|
Local Mediators |
|||
|
Epidermal growth factor (EGF) |
Various cells |
Protein |
Stimulates epidermal and many other cell types to proliferate |
|
Platelet-derived growth factor (PDGF) |
Various cells, including blood platelets |
Protein |
Stimulates many cell types to proliferate |
|
Nerve growth factor (NGF) |
Various innervated tissues |
Protein |
Promotes survival and axonal growth of certain classes of neurons |
|
Histamine |
Mast cells |
Derivative of the amino acid histidine |
Causes blood vessels to dilate and become leaky, helping to cause inflammation |
|
Nitric oxide (NO) |
Nerve cells; endothelial cells lining blood vessels |
Dissolved gas |
Causes smooth muscle cells to relax; regulates nerve-cell activity |
|
Neurotransmitters |
|||
|
Acetylcholine |
Nerve terminals |
Derivative of choline |
Excitatory neurotransmitter at many nerve–muscle synapses and in central nervous system |
|
γ-Aminobutyric acid (GABA) |
Nerve terminals |
Derivative of the amino acid glutamic acid |
Inhibitory neurotransmitter in central nervous system |
|
Contact-dependent Signal Molecules |
|||
|
Delta |
Prospective neurons; various other developing cell types |
Transmembrane protein |
Inhibits neighboring cells from becoming specialized in the same way as the signaling cell |
A typical cell in a multicellular organism is exposed to hundreds of different signal molecules in its environment. These may be free in the extracellular fluid, embedded in the extracellular matrix in which many cells reside, or bound to the surface of neighboring cells. Each cell must respond very selectively to this mixture of signals, disregarding some and reacting to others, according to the cell’s specialized function.
QUESTION 16–1
To keep their action local, paracrine signal molecules must be prevented from straying too far from their points of origin. Suggest different ways by which this could be accomplished. Explain your answers.
Whether a cell responds to a signal molecule depends, first of all, on whether it possesses a receptor for that signal. Each receptor is usually activated by only one type of signal. Without the appropriate receptor, a cell will be deaf to the signal and will not respond to it.
Extracellular signal molecules can be divided into two major classes, depending on the type of receptor with which they interact. The first and largest class of signals consists of molecules that are too large or too hydrophilic to cross the plasma membrane of the target cell. These signal molecules rely on receptors on the surface of the target cell to relay their message across the plasma membrane (Figure 16–4A). The second class of signals consists of molecules that are small enough or hydrophobic enough to pass through the plasma membrane and into the cytosol of the target cell, where they bind to intracellular receptor proteins (Figure 16–4B). In this chapter, we focus primarily on signaling through cell-surface receptors, but we will briefly describe signaling through intracellular receptors in the final section.
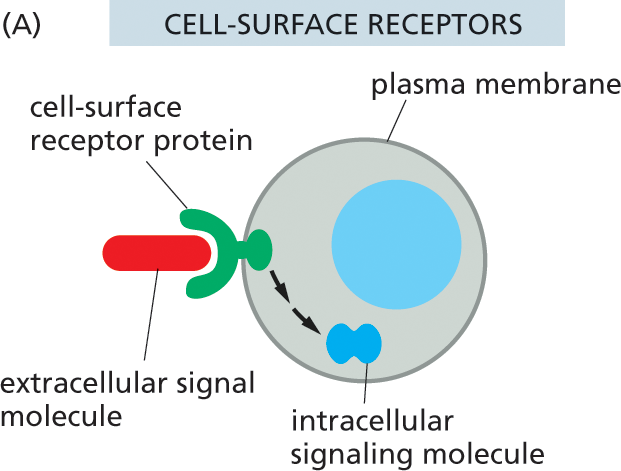
Illustration A shows signal molecules binding to the receptors on the outer surface of the cell. A rounded cell is shown. The periphery is labeled plasma membrane. A pair of small oval structures below the nucleus are labeled intracellular signaling molecule. An extracellular signal molecule binds to the cell surface receptor protein on the outside of the cell and is then converted to an intracellular signaling molecule.
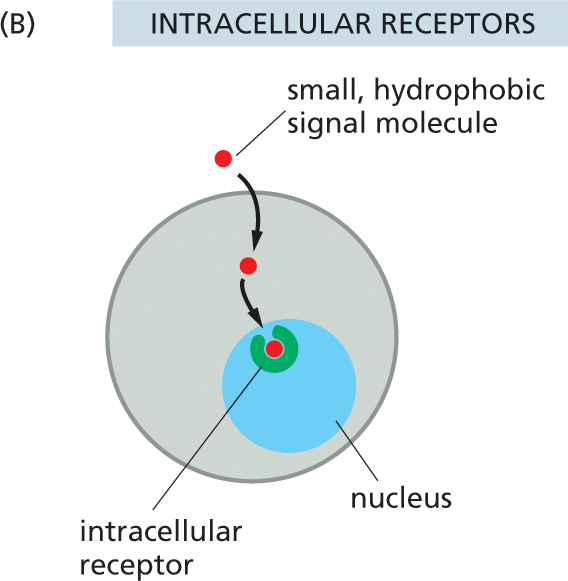
Illustration B shows signal molecules binding to the receptors inside the cell. A rounded cell with an intracellular receptor labeled inside the nucleus is shown. An extracellular small hydrophobic signal molecule enters the cell through the plasma membrane, and is transmitted to the intracellular receptor in the nucleus of the cell.
By producing a limited set of receptors—out of the thousands that are possible—a cell restricts the types of signals that can affect it. However, even a restricted set of extracellular signal molecules can change the behavior of a target cell in a tremendous variety of ways, altering its shape, movement, metabolism, gene expression, or some combination of these. How a cell reacts to a particular signal depends on the set of intracellular signaling molecules each cell-surface receptor produces and how these molecules alter the activity of effector proteins, which have a direct effect on the behavior of the target cell. This intracellular relay system and the intracellular effector proteins on which it acts vary from one type of specialized cell to another, so that different types of cells respond to the same signal in different ways. For example, when a heart pacemaker cell is exposed to the neurotransmitter acetylcholine, its rate of firing decreases. When a salivary gland cell is exposed to the same signal, it secretes components of saliva, even though the receptors on both cell types are the same. In skeletal muscle, acetylcholine binds to a different receptor protein, causing the muscle cell to contract (Figure 16–5). Thus, the extracellular signal molecule alone is not the message: the information conveyed by the signal depends on how the target cell receives and interprets the signal.
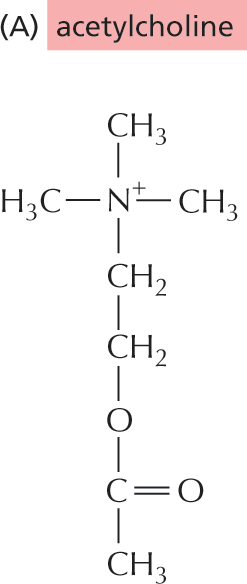
Illustration A shows the chemical structure of acetylcholine. The structure shows a positively charged nitrogen single bonded to three methyl groups and a methylene group. The methylene group is single bonded to another methylene group which is further single bonded to an oxygen atom. The oxygen atom is single bonded to a carbon atom below. The carbon is double bonded to another oxygen atom and single bonded to a methyl group.
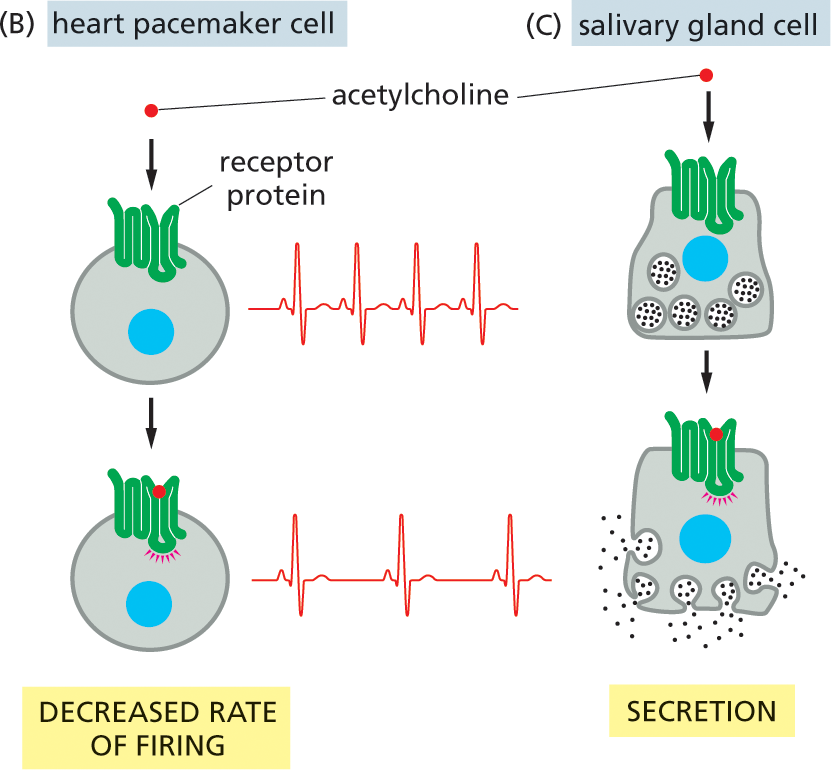
Illustration B depicts decreased rate of firing in a heart pacemaker cell in response to the signal from acetylcholine. A targeted heart pacemaker cell with a nucleus and a receptor protein bound to its surface is shown, with a more rapid heart rate electrocardiogram shown to the right. An acetylcholine molecule is shown moving toward the receptor protein. Below, the acetylcholine is shown bound to the receptor protein, with a slower heart rate electrocardiogram shown to the right.; Illustration C depicts the response of a salivary gland cell to the signal from acetylcholine. An irregularly shaped cell with the nucleus, receptor protein on the outside, and saliva within the cell is shown. Acetylcholine moves toward the receptor protein in the first step. In the next step, the acetylcholine molecule binds to the receptor protein, and saliva shown being secreted from the glands.
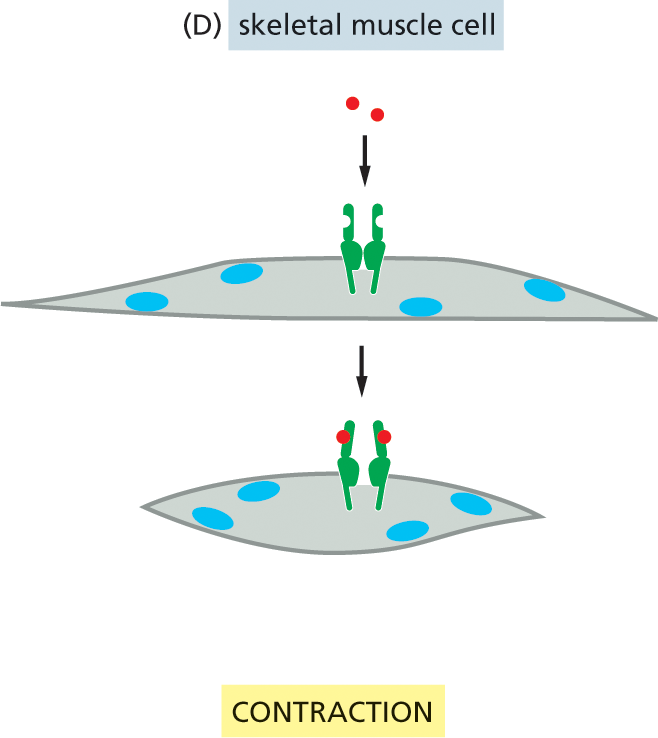
Illustration D depicts muscle cell contraction in response to acetylcholine binding to the receptor protein. Acetylcholine molecules move toward an open receptor on the surface of a skeletal muscle cell in the first step. In the next step, the acetylcholine molecule binds to the receptor protein, and the cell contracts.
A typical cell possesses many sorts of receptors—each present in tens to hundreds of thousands of copies. Such variety makes the cell simultaneously sensitive to many different extracellular signals and allows a relatively small number of signal molecules, used in different combinations, to exert subtle and complex control over cell behavior. A combination of signals can evoke a response that is different from the sum of the effects that each signal would trigger on its own. As we discuss later, this “tailoring” of a cell’s response occurs, in part, because the intracellular relay systems activated by the different signals interact. Thus the presence of one signal will often modify the effects of another. One combination of signals might enable a cell to survive; another might drive it to differentiate in some specialized way; and another might cause it to divide. In the absence of the proper signals, most animal cells are programmed to undergo apoptosis, a form of cell suicide (Figure 16–6).
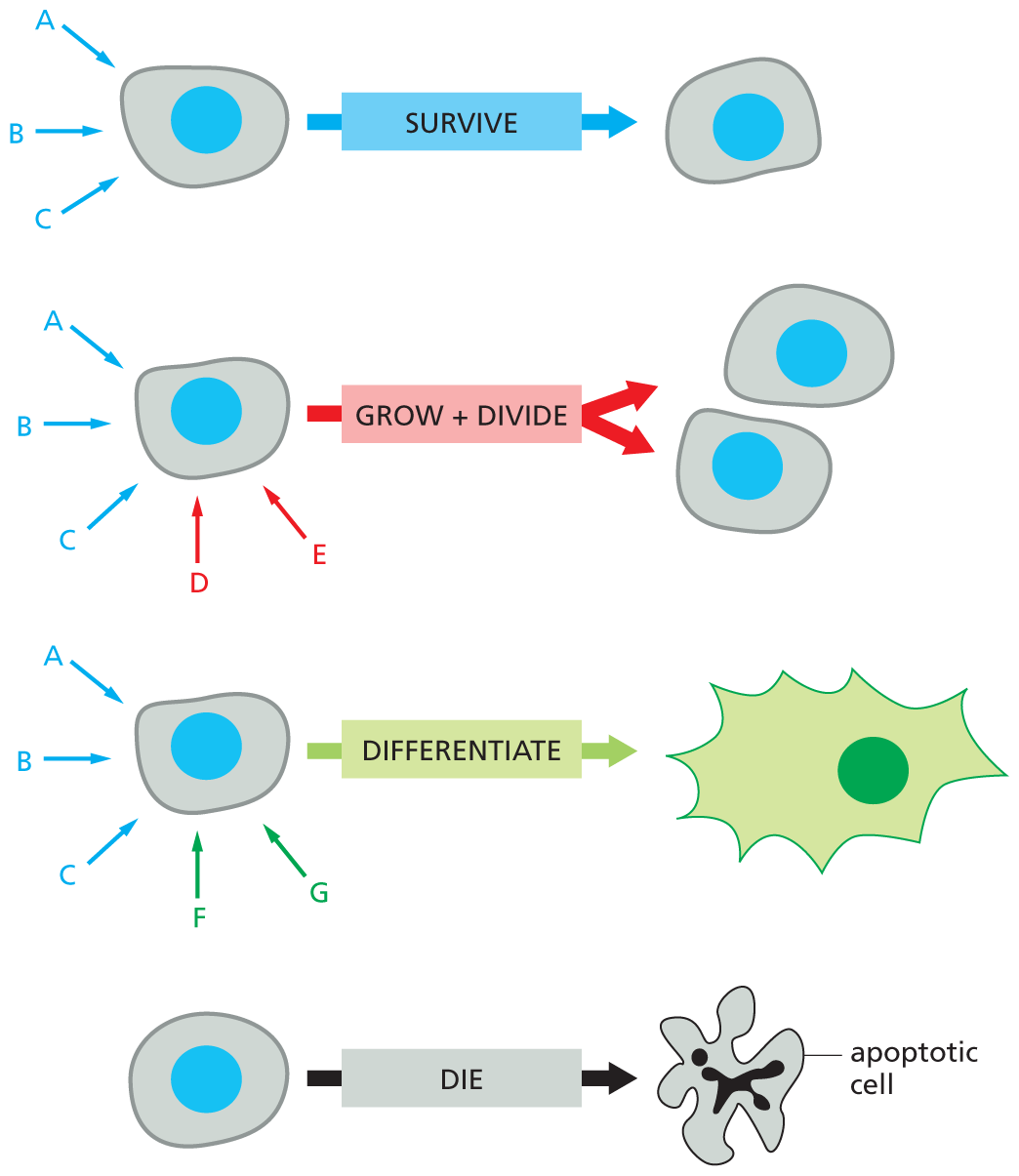
An illustration depicts different types of animal cells responding to extracellular signals. The first cell receives three extracellular signals A, B, and C in blue, and the cell continues to survive in response. The second cell receives signals A, B, C, D, and E grows and divides to form two cells of the same type. Signals D and E are in red, A, B and C, are in blue. The third cell receives five signals, A, B, C, F, and G. It differentiates to form an irregularly shaped cell. Signals F and G are in green, A, B, and C are in blue. The last cell receives no extracellular signals, and dies to form an apoptotic cell.
Figure 16–6 An animal cell depends on multiple extracellular signals. Every cell type displays a set of receptor proteins that enables it to respond to a specific set of extracellular signal molecules produced by other cells. These signal molecules work in combinations to regulate the behavior of the cell. As shown here, cells may require multiple signals (blue arrows) to survive, additional signals (red arrows) to grow and divide, and still other signals (green arrows) to differentiate. If deprived of the necessary survival signals, most cells undergo a form of cell suicide known as apoptosis (discussed in Chapter 18).
The length of time a cell takes to respond to an extracellular signal can vary greatly, depending on what needs to happen once the message has been received. Some extracellular signals act swiftly: acetylcholine can stimulate a skeletal muscle cell to contract within milliseconds and a salivary gland cell to secrete within a minute or so. Such rapid responses are possible because, in each case, the signal affects the activity of proteins that are already present inside the target cell, awaiting their marching orders.
Other responses take more time. Cell growth and cell division, when triggered by the appropriate signal molecules, can take many hours to execute. This is because the response to these extracellular signals requires changes in gene expression and the production of new proteins (Figure 16–7). We will encounter additional examples of both fast and slow responses—and the signal molecules that stimulate them—throughout this chapter.
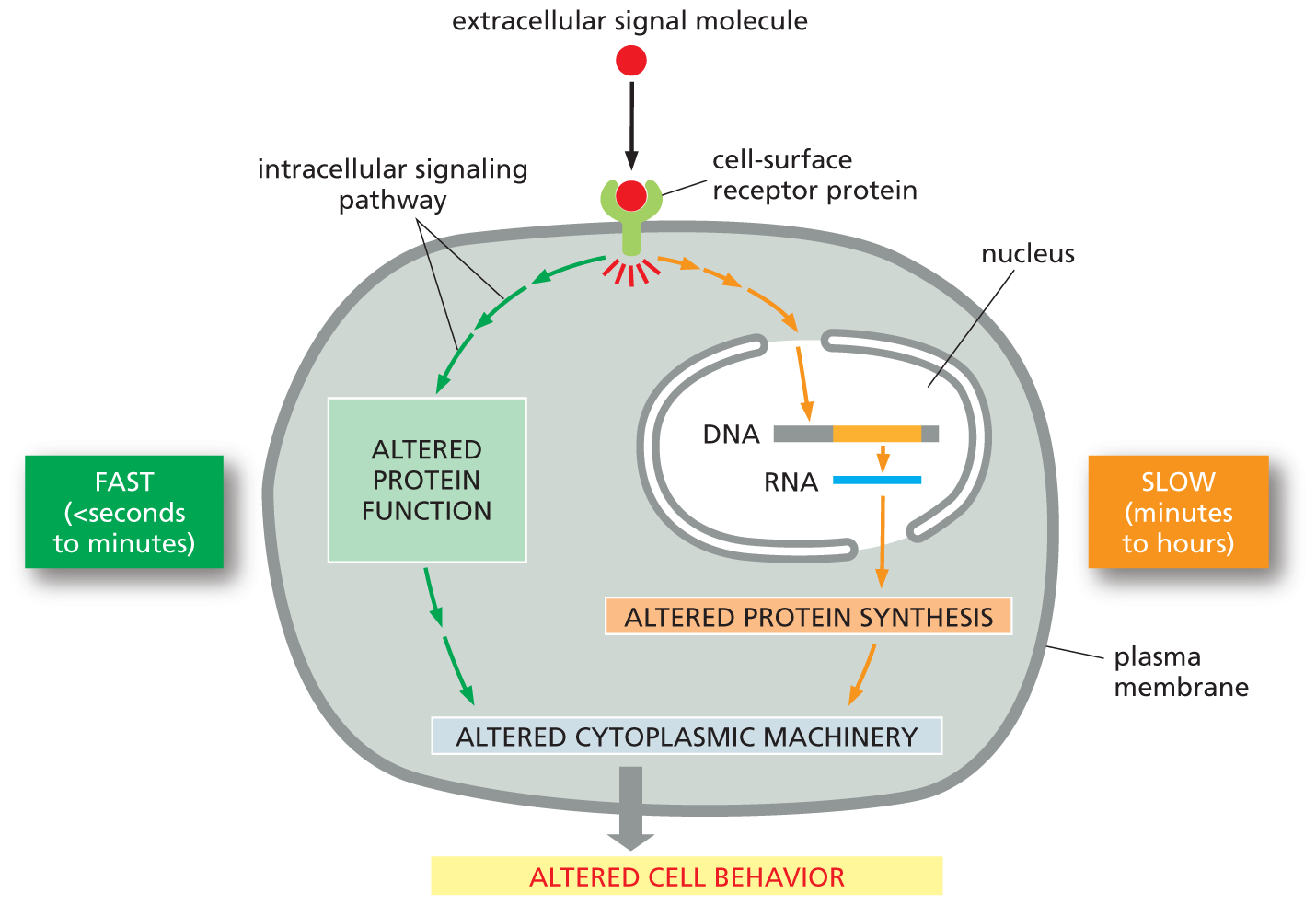
An illustration shows pathways by which extracellular signals act slowly or rapidly. The process begins with an extracellular signal molecule binding to a cell-surface receptor protein. Two pathways are shown within the cell, with the left side being the fast response (seconds to minutes), and the right side being the slow response (minutes to hours).
On the fast (seconds to minutes) side, the signal from the cell-surface receptor protein is passed on to an altered protein function block through an intracellular signaling pathway. This leads to altered cytoplasmic machinery, which induces altered cell behavior.
On the slow (minutes to hours) side, the signal from the receptor protein is passed on to the nucleus inside the cell which moves from a D N A strand to an R N A strand. The signal emerging out from the nucleus leads to altered protein synthesis and then altered cytoplasmic machinery, which induces altered cell behavior.
Figure 16–7 Extracellular signals can act slowly or rapidly. Certain types of cell responses—such as cell differentiation or increased cell growth and division (see Figure 16–6)—involve changes in gene expression and the synthesis of new proteins; they therefore occur relatively slowly. Other responses—such as changes in cell movement, secretion, or metabolism—need not involve changes in gene expression and therefore occur more quickly (see Figure 16–5).
The majority of extracellular signal molecules are proteins, peptides, or small, hydrophilic molecules that bind to cell-surface receptors that span the plasma membrane (see Figure 16–4A). Transmembrane receptors detect a signal on the outside and relay a message across the membrane into the interior of the cell.
The receptor protein performs the primary step in signal transduction: it recognizes an extracellular signal molecule and generates a different type of intracellular signal molecule in response (see Figure 16–2B). The resulting intracellular signaling process usually works like a molecular relay race, in which the message is passed “downstream” from one intracellular signaling molecule to another, each activating or generating the next signaling molecule in the pathway, until a metabolic enzyme is kicked into action, the cytoskeleton is tweaked into a new configuration, or a gene is switched on or off. This final outcome is called the response of the cell (Figure 16–8).
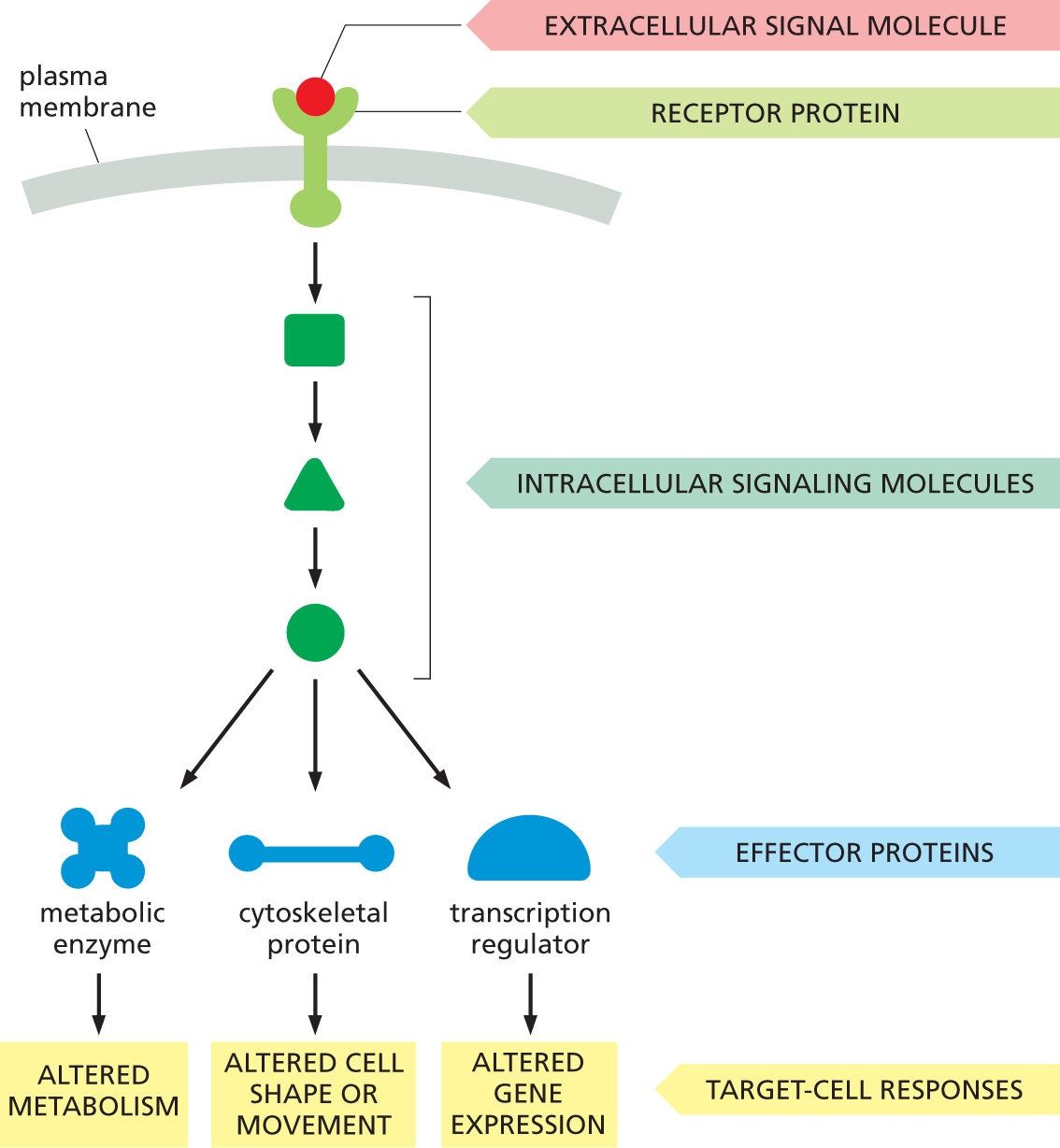
A flow diagram shows how extracellular signals influence the behavior of a target cell. An extracellular signal molecule binds to a receptor protein on the plasma membrane, and intracellular signaling pathways are activated. The intracellular signaling molecules act upon three effector proteins - metabolic enzyme, cytoskeletal protein, and transcription regulator, to generate different target cell responses. The effect on a metabolic enzyme is to cause altered metabolism, on a cytoskeletal protein, to cause altered cell shape or movement, and on transcription regulators, to cause altered gene expression.
Figure 16–8 Many extracellular signals activate intracellular signaling pathways to change the behavior of the target cell. A cell-surface receptor protein activates one or more intracellular signaling pathways, each mediated by a series of intracellular signaling molecules, which can be proteins or small messenger molecules. Signaling molecules eventually interact with specific effector proteins, altering them to change the behavior of the cell in various ways.
QUESTION 16–2
In principle, how might an intracellular signaling protein amplify a signal as it relays it onward?
The components of these intracellular signaling pathways perform one or more crucial functions (Figure 16–9):
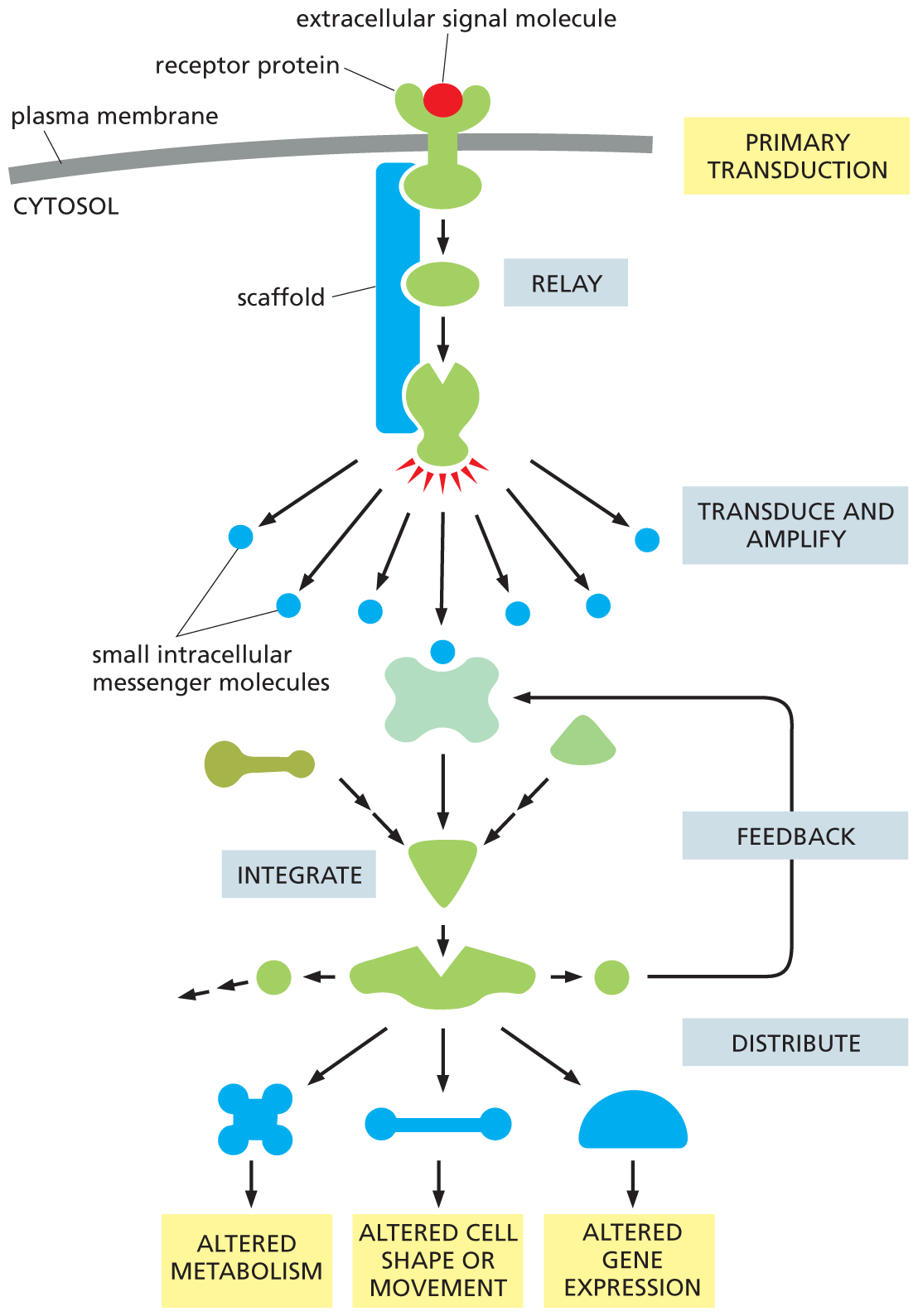
A flow diagram depicts the signaling pathway of an intracellular signaling protein. An extracellular signal molecule binds with a receptor protein on the plasma membrane in primary transduction, and passes through a scaffold comprising two protein molecules. This transmission is termed relay. The second protein molecule produces signals which are passed onto small intracellular messenger molecules. This process is called transduce and amplify. These molecules are integrated to form one unit which leads to two other molecules. The second molecule produces two other molecules of which one enters the feedback loop. The second unit distributes signals to generate three types of target cell responses: altered metabolism, altered cell shape or movement, and altered gene expression.
Figure 16–9 Intracellular signaling proteins can relay, amplify, integrate, distribute, and modulate via feedback an incoming signal. In this example, a receptor protein located on the cell surface transduces an extracellular signal into an intracellular signal, which initiates one or more intracellular signaling pathways that relay the signal into the cell interior. Each pathway includes intracellular signaling proteins that can function in one of the various ways shown; some, for example, integrate signals from other intracellular signaling pathways. Many of the steps in the process can be modulated via feedback by other molecules or events in the cell. Note that some proteins in the pathway may be held in close proximity by a scaffold protein, which allows them to be activated at a specific location in the cell and with greater speed, efficiency, and selectivity (discussed in Chapter 4; see Figure 4–51). We review the production and function of small intracellular messenger molecules, more commonly called second messenger molecules, later in this chapter.
Although feedback regulation is last on our list, it is actually a very important feature of cell signaling. Feedback can occur anywhere in the signaling pathway and can either boost or weaken the response to the signal. In positive feedback, a component that lies downstream in the pathway acts on an earlier component in the same pathway to enhance the response to the initial signal; in negative feedback, a downstream component acts to inhibit an earlier component in the pathway to diminish the response to the initial signal (Figure 16–10). Such feedback regulation is very common in biological systems and can lead to sophisticated responses: positive feedback can generate all-or-none, switchlike responses, for example, whereas negative feedback can generate responses that oscillate on and off as the activities or concentrations of the inhibitory components rise and fall.
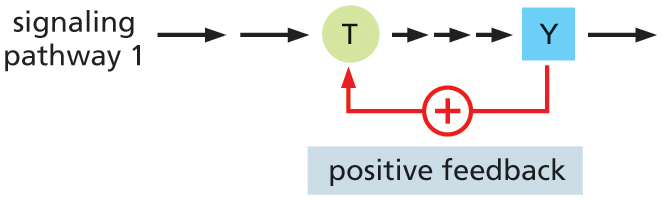
Illustration A shows the positive feedback regulation process in intracellular signaling. Signaling pathway 1 has two long arrows pointing to protein T, and three short arrows from T to protein Y. An arrow is drawn from protein Y to T, indicating positive feedback.
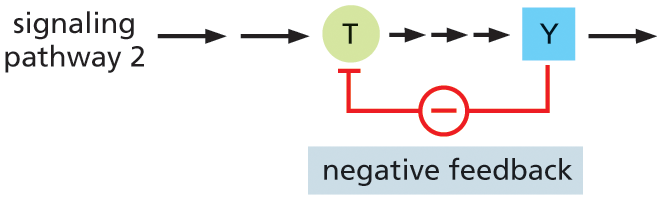
Illustration B shows the negative feedback regulation process in intracellular signaling. Signaling pathway 2 has two long arrows pointing to protein T, and three short arrows from T to protein Y. An arrow is drawn from protein Y to T, indicating negative feedback.
Many intracellular signaling proteins behave as molecular switches: receipt of a signal causes them to switch from an inactive to an active state. Once activated, these proteins can stimulate—or in some cases suppress—other proteins in the signaling pathway. They then persist in an active state until some other process switches them off again.
The importance of the switching-off process is often underappreciated: imagine the consequences if a signaling pathway that boosts your heart rate were to remain active indefinitely. If a signaling pathway is to recover after transmitting a signal and make itself ready to transmit another, every activated protein in the pathway must be reset to its original, unstimulated state. Thus, for every activation step along the pathway, there exists an inactivation mechanism. The two are equally important for a signaling pathway to be useful—and to allow the process to be regulated.
Proteins that act as molecular switches fall mostly into one of two classes. The first—and by far the largest—class consists of proteins that are activated or inactivated by phosphorylation, a chemical modification discussed in Chapter 4 (see Figure 4–44). For these molecules, the switch is thrown in one direction by a protein kinase, which covalently attaches a phosphate group onto the switch protein, and in the opposite direction by a protein phosphatase, which takes the phosphate off again (Figure 16–11A). The activity of any protein that is regulated by phosphorylation depends—moment by moment—on the balance between the activities of the protein kinases that phosphorylate it and the protein phosphatases that dephosphorylate it.
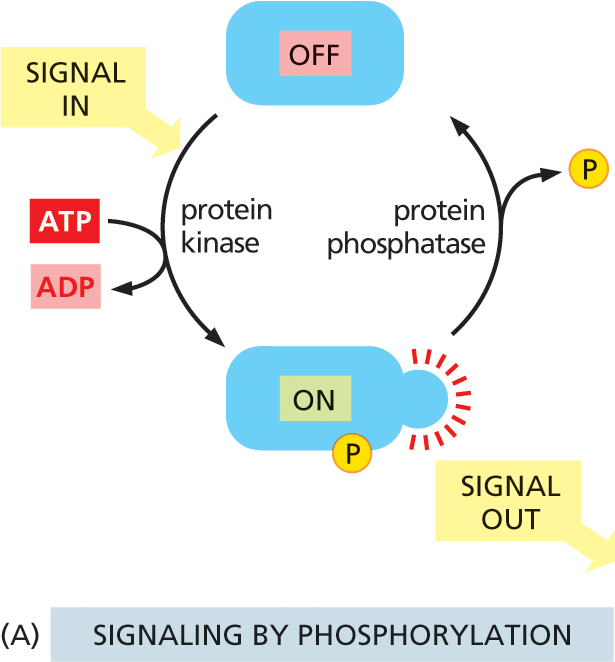
Illustration A shows the cyclic process of signaling by protein phosphorylation. A protein is shown to be in the OFF state. Input signal causes protein kinase to bond the phosphate which is released from the conversion of A T P to A D P, to a protein at the bottom, which is turned ON. This protein transmits out signals. Protein phosphatase releases the phosphate in the next step shifting the protein to OFF state, and the cycle continues.
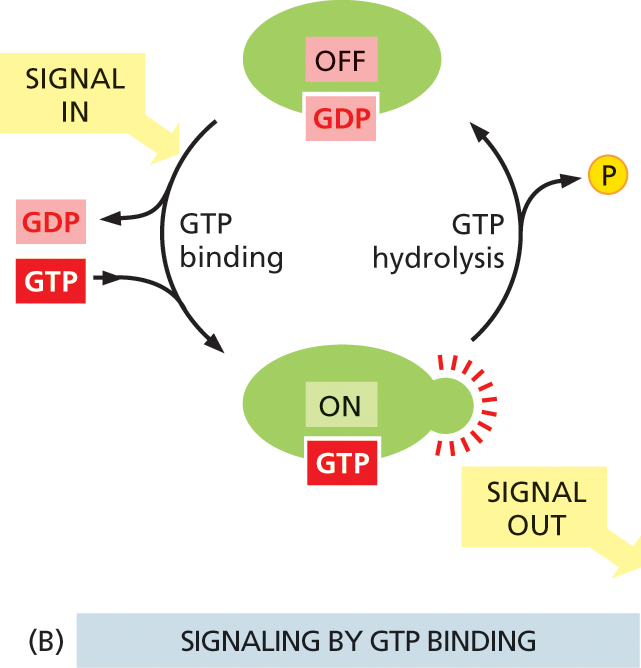
Illustration B shows cyclic process of signaling by protein phosphorylation. A G D P bound protein is shown to be in the OFF state at the top. An input signal causes G T P binding and release of G D P. The protein is now bound to G T P, is in ON state and transmits out signals. This G T P bound protein undergoes G T P hydrolysis and releases the phosphate. The protein is now bound to G D P and is in the OFF state.
Many of the switch proteins controlled by phosphorylation are themselves protein kinases, and these are often organized into phosphorylation cascades: one protein kinase, activated by phosphorylation, phosphorylates the next protein kinase in the sequence, and so on, transmitting the signal onward and, in the process, amplifying, distributing, and regulating it. Two main types of protein kinases operate in intracellular signaling pathways: the most common are serine/threonine kinases, which—as the name implies—phosphorylate proteins on serines or threonines; others are tyrosine kinases, which phosphorylate proteins on tyrosines.
The other major class of switch proteins involved in intracellular signaling pathways consists of GTP-binding proteins. These toggle between an active and an inactive state depending on whether they have GTP or GDP bound to them, respectively (Figure 16–11B). Once activated by GTP binding, many of these proteins have intrinsic GTP-hydrolyzing (GTPase) activity, and they shut themselves off by hydrolyzing their bound GTP to GDP.
Two main types of GTP-binding proteins participate in intracellular signaling. The first type—the large, trimeric GTP-binding proteins (also called G proteins)—relay messages from G-protein-coupled receptors. We discuss this major class of GTP-binding proteins in detail shortly.
Other cell-surface receptors rely on a second type of GTP-binding protein—the small, monomeric GTPases—to help relay their signals. These switch proteins are generally aided by two sets of regulatory proteins that help them bind and hydrolyze GTP: guanine nucleotide exchange factors (GEFs) activate the switches by promoting the exchange of GDP for GTP, and GTPase-activating proteins (GAPs) turn them off by promoting GTP hydrolysis (Figure 16–12). We will return to this type of GTP-binding protein later in this chapter, when we discuss the enzyme-coupled receptors that rely on these switches to propagate their message.
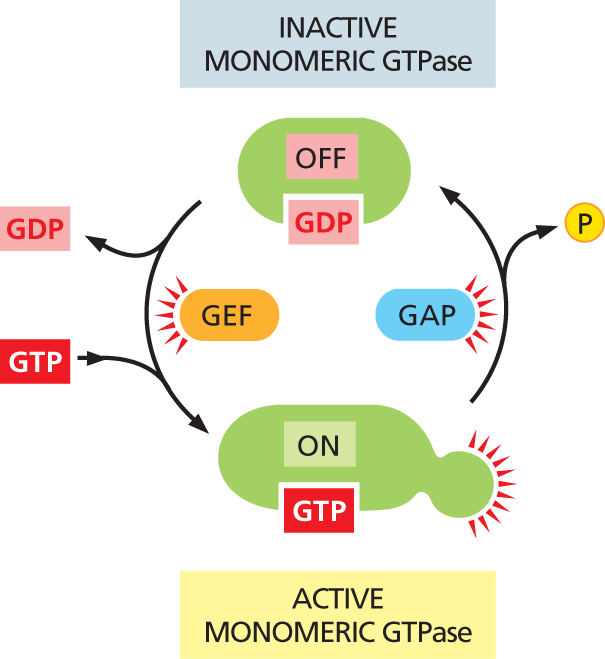
An illustration of a cyclic flowchart depicts signal transfer between inactive and active monomeric G T P ase. A G D P bound protein (inactive monomeric G T P ase) is shown to be in the OFF state at the top. Signals from G E F release G D P and G T P binds to the protein, turning the configuration to the ON state (active monomeric G T P ase). The active monomeric G T P ase transmits signals. In the next step, G A P causes the release of phosphate and the protein is bound to G D P again and is in the OFF state.
Figure 16–12 The activity of monomeric GTPases is controlled by two types of regulatory proteins. Guanine nucleotide exchange factors (GEFs) promote the exchange of GDP for GTP, thereby switching the protein on. The protein is turned off by hydrolysis of GTP to GDP, which is stimulated by GTPase-activating proteins (GAPs).
All cell-surface receptor proteins bind to an extracellular signal molecule and transduce its message into one or more intracellular signaling molecules that alter the cell’s behavior. Most of these receptors belong to one of three large classes, which differ in the transduction mechanism they use.
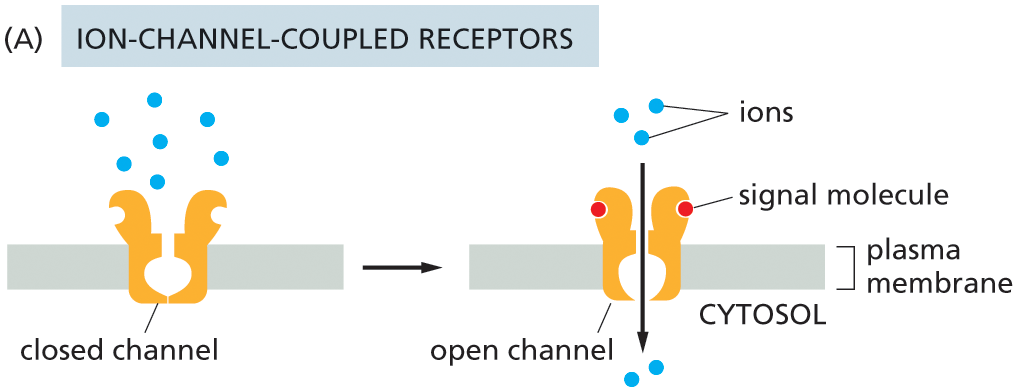
Illustration A shows the function of ion-channel coupled receptors. A closed channel is shown attached to the plasma membrane with ions on the extracellular side of the channel. In the next stage, the channel opens, two signal molecules bind to the channel on the extracellular side, and the ions pass through the channel into the cytosol.
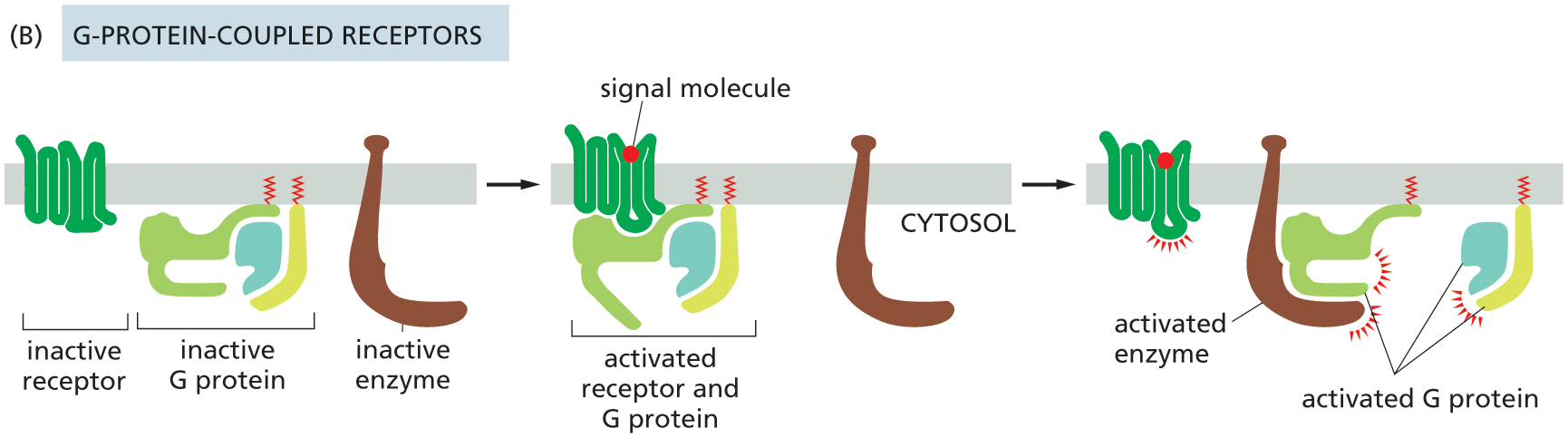
Illustration B shows the function of G-protein coupled receptors. An inactive receptor protein, inactive G protein and an inactive enzyme are attached to the plasma membrane. In the next stage, a signal molecule is bound to the inactive receptor and the activated receptor binds to G protein in the cytosol. In the final stage, the signal molecule bound to the activated receptor remains separately attached to the plasma membrane, and the activated enzyme is bound to activated G protein.
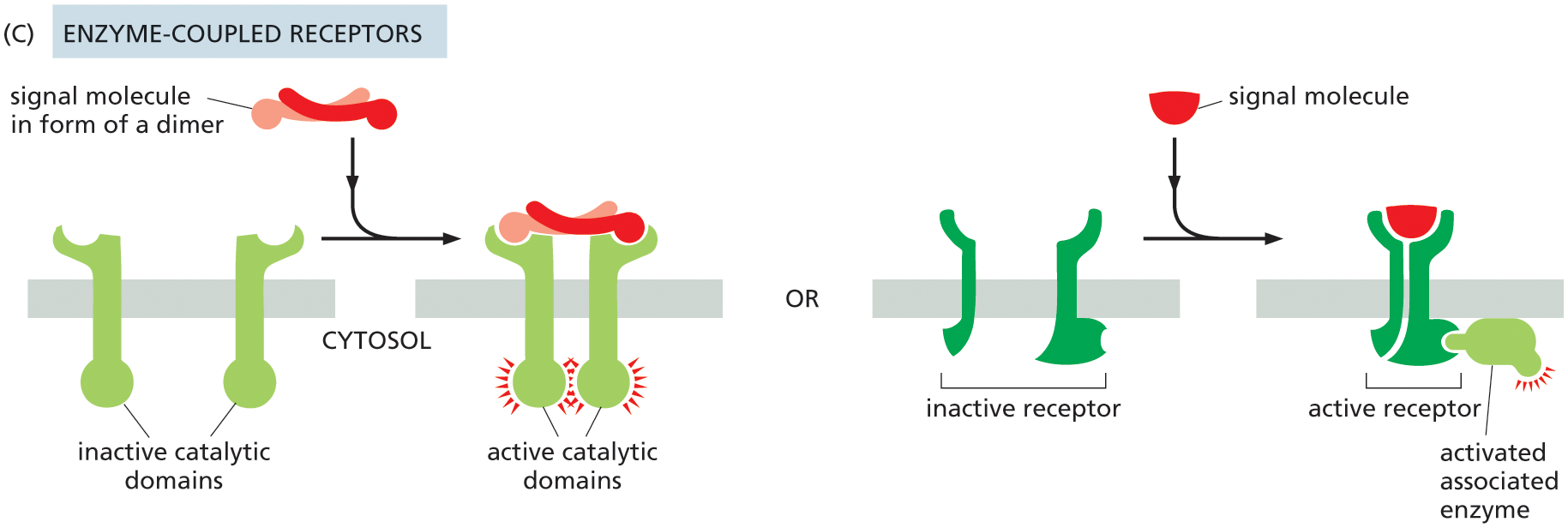
Illustration C shows the function of enzyme-coupled receptors. Two types are illustrated. A pair of inactive catalytic domains are attached to the plasma membrane initially. A signal molecule in the form of a dimer binds with the inactive catalytic domain outside of the cell to form active catalytic domains inside of the cell.
In the other method part of the illustration, a pair of inactive receptors are attached to the plasma membrane initially. A signal molecule binds with the inactive receptor parts to bring them together and form an active receptor which is bound to an activated associated enzyme on the cytosolic side.
The number of different types of receptors in each of these three classes is even greater than the number of extracellular signals that act on them. This is because for many extracellular signal molecules there is more than one type of receptor, and these may belong to different receptor classes. The neurotransmitter acetylcholine, for example, acts on skeletal muscle cells via an ion-channel-coupled receptor, whereas in heart cells it acts through a G-protein-coupled receptor. These two types of receptors generate different intracellular signals and thus enable the two types of cells to react to acetylcholine in different ways, increasing contraction in skeletal muscle and decreasing the rate of contractions in the heart (see Figure 16–5A and C).
This extensive assortment of cell-surface receptors also provides targets for many foreign substances that interfere with our physiology, from heroin and nicotine to tranquilizers and chili peppers. These substances can overstimulate—or block—the receptor’s natural activity. Many drugs and poisons act in this way (Table 16–2), and a large part of the pharmaceutical industry is devoted to producing drugs that will exert a precisely defined effect by binding to a specific type of cell-surface receptor.
|
TABLE 16–2 SOME FOREIGN SUBSTANCES THAT ACT ON CELL-SURFACE RECEPTORS |
|||
|
Substance |
Normal Signal |
Receptor Action |
Effect |
|
Barbiturates and benzodiazepines (Valium and Ambien) |
γ-Aminobutyric acid (GABA) |
Stimulate GABA-activated ion-channel-coupled receptors |
Relief of anxiety; sedation |
|
Nicotine |
Acetylcholine |
Stimulates acetylcholine-activated ion-channel-coupled receptors |
Constriction of blood vessels; elevation of blood pressure |
|
Morphine and heroin |
Endorphins and enkephalins |
Stimulate G-protein-coupled opiate receptors |
Analgesia (relief of pain); euphoria |
|
Curare |
Acetylcholine |
Blocks acetylcholine-activated ion-channel-coupled receptors |
Blockage of neuromuscular transmission, resulting in paralysis |
|
Strychnine |
Glycine |
Blocks glycine-activated ion-channel-coupled receptors |
Blockage of inhibitory synapses in spinal cord and brain, resulting in seizures and muscle spasm |
|
Capsaicin |
Heat |
Stimulates temperature-sensitive ion-channel-coupled receptors |
Painful, burning sensation; prolonged exposure can paradoxically lead to pain relief |
|
Menthol |
Cold |
Stimulates temperature-sensitive ion-channel-coupled receptors |
In moderate amounts, a cool sensation; at higher doses, can cause burning pain |
Of all the types of cell-surface receptors, ion-channel-coupled receptors (also known as transmitter-gated ion channels) function in the simplest and most direct way. As we discuss in detail in Chapter 12, these receptors are responsible for the rapid transmission of signals across synapses in the nervous system. They transduce a chemical signal, in the form of a pulse of secreted neurotransmitter molecules delivered to a target cell, directly into an electrical signal, in the form of a change in voltage across the target cell’s plasma membrane (see Figure 12–43). When the neurotransmitter binds to ion-channel-coupled receptors on the surface of a target cell, the receptor alters its conformation so as to open a channel in the target cell membrane, rendering it permeable to specific types of ions, such as Na+, K+, or Ca2+ (see Figure 16–13A and Movie 16.1). Driven by their electrochemical gradients, the ions rush into or out of the cell, creating a change in the membrane potential within milliseconds. This change in potential may trigger a nerve impulse or make it easier (or harder) for other neurotransmitters to do so. As we discuss later, the opening of Ca2+ channels has additional important effects, as changes in the Ca2+ concentration in the target-cell cytosol can profoundly alter the activities of many Ca2+-responsive proteins.
Whereas ion-channel-coupled receptors are especially important in nerve cells and other electrically excitable cells such as muscle cells, G-protein-coupled receptors and enzyme-coupled receptors are important for practically every cell type in the body. Most of the remainder of this chapter deals with these two receptor families and with the signal transduction processes that they use.