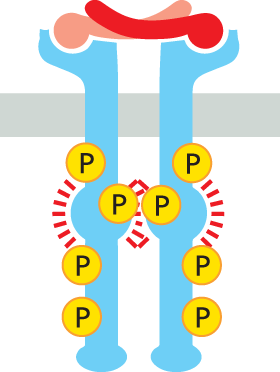
QUESTION 16–6
One important feature of any intracellular signaling pathway is its ability to be turned off. Consider the pathway shown in Figure 16–28. Where would off switches be required? Which ones do you suppose would be the most important?
Like GPCRs, enzyme-coupled receptors are transmembrane proteins that display their ligand-binding domains on the outer surface of the plasma membrane (see Figure 16–13C). Instead of associating with a G protein, however, the cytoplasmic domain of the receptor either acts as an enzyme itself or forms a complex with another protein that acts as an enzyme. Enzyme-coupled receptors were discovered through their role in responses to extracellular signal proteins that regulate the growth, proliferation, differentiation, and survival of cells in animal tissues (see Table 16–1, p. 556, for examples). Most of these signal proteins function as local mediators and can act at very low concentrations (about 10–9 to 10–11 M). Responses to them are typically slow (on the order of hours), and their effects may require many intracellular transduction steps that usually lead to a change in gene expression.
QUESTION 16–6
One important feature of any intracellular signaling pathway is its ability to be turned off. Consider the pathway shown in Figure 16–28. Where would off switches be required? Which ones do you suppose would be the most important?
The largest class of enzyme-coupled receptors consists of receptors with a cytoplasmic domain that functions as a tyrosine kinase, which phosphorylates particular tyrosines on specific intracellular signaling proteins. These receptors, called receptor tyrosine kinases (RTKs), will be the main focus of this section.
We begin with a discussion of how RTKs are activated in response to extracellular signals. We then consider how activated RTKs transmit the signal along two major intracellular signaling pathways that terminate at various effector proteins in the target cell. Finally, we describe how some enzyme-coupled receptors bypass such intracellular signaling cascades and use a more direct mechanism to regulate gene transcription.
Abnormal cell growth, proliferation, differentiation, survival, and migration are fundamental features of a cancer cell, and abnormalities in signaling via RTKs and other enzyme-coupled receptors have a major role in the development of most cancers.
To do its job as a signal transducer, an enzyme-coupled receptor has to switch on the enzyme activity of its intracellular domain (or of an associated enzyme) when an external signal molecule binds to its extracellular domain. Unlike GPCRs, enzyme-coupled receptor proteins usually have only one transmembrane segment, which spans the lipid bilayer as a single α helix. Because a single α helix is poorly suited to transmit a conformational change across the bilayer, enzyme-coupled receptors have a different strategy for transducing the extracellular signal. In many cases, the binding of an extracellular signal molecule causes two receptor molecules to come together in the plasma membrane, forming a dimer. This pairing brings the two intracellular tails of the receptors together and activates their kinase domains, such that each receptor tail phosphorylates the other. In the case of RTKs, the phosphorylations occur on specific tyrosines.
This tyrosine phosphorylation then triggers the assembly of a transient but elaborate intracellular signaling complex on the cytosolic tails of the receptors. The newly phosphorylated tyrosines serve as docking sites for a whole zoo of intracellular signaling proteins—perhaps as many as 10 or 20 different molecules (Figure 16–29). Some of these proteins become phosphorylated and activated on binding to the receptors, and they then propagate the signal; others function solely as scaffolds, which couple the receptors to other signaling proteins, thereby helping to build the active signaling complex.
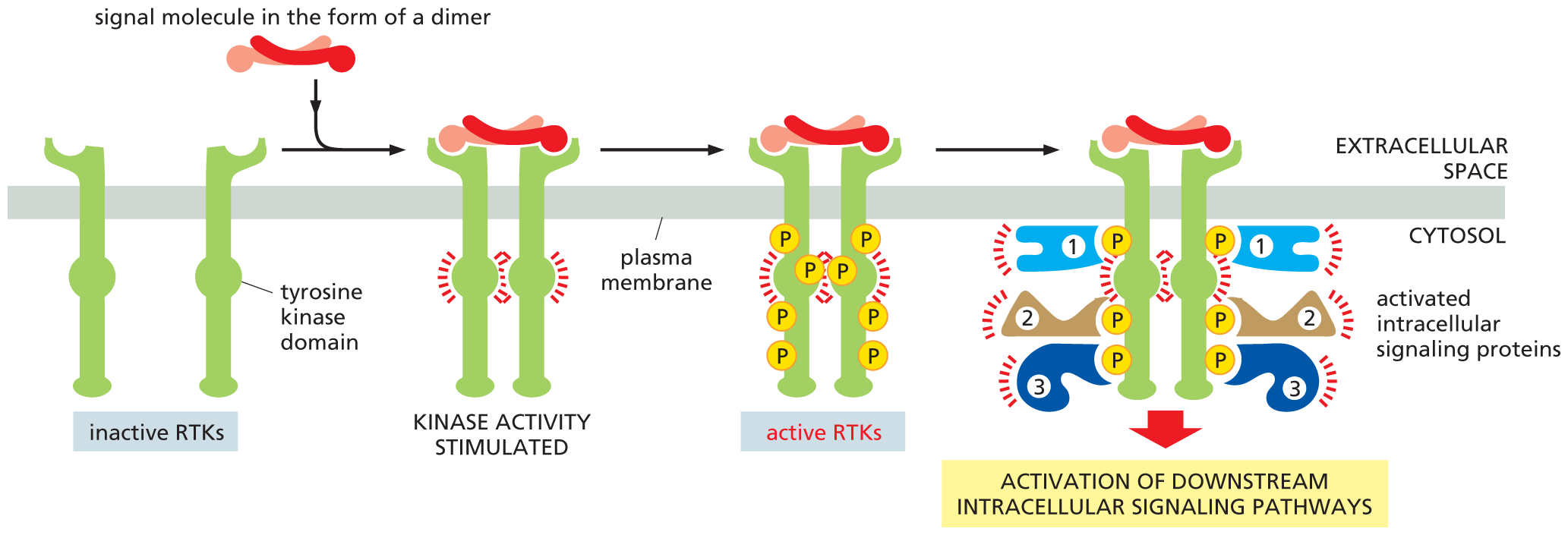
An illustration shows how activation of an R T K leads to the formation of an intracellular signaling complex. Inactive R T K with a tyrosine kinase domain binds to a signal molecule in the form of a dimer. The signal molecule brings the two inactive R T Ks together. This stimulates kinase activity in the tyrosine kinase domain of R T K. Active R T K forms in the next step. The schematic shows many phosphates bound to the tyrosine kinase domain of the R T Ks on the cytosolic side. In the next step, each of these phosphates activate intracellular signaling proteins (numbered 1, 2, and 3) leading to the activation of downstream intracellular signaling pathways in the cytosol.
Figure 16–29 Activation of an RTK stimulates the assembly of an intracellular signaling complex. Typically, the binding of a signal molecule to the extracellular domain of an RTK causes two receptor molecules to associate into a dimer. The signal molecule shown here is itself a dimer and thus can physically cross-link two receptor molecules; other signal molecules induce a conformational change in the RTKs, causing the receptors to dimerize (not shown). In either case, dimer formation brings the kinase domain of each cytosolic receptor tail into contact with the other; this activates the kinases to phosphorylate several tyrosines on the adjacent receptor tail. Each phosphorylated tyrosine serves as a specific docking site for a different intracellular signaling protein, which then helps relay the signal to the cell’s interior; these proteins contain a specialized interaction domain—in this case, a module called an SH2 domain—that recognizes and binds to specific phosphorylated tyrosines on the cytosolic tail of an activated RTK or on another intracellular signaling protein.
All of these docked intracellular signaling proteins possess a specialized interaction domain, which recognizes specific phosphorylated tyrosines on the receptor tails. A different interaction domain allows intracellular signaling proteins to recognize phosphorylated lipids that are produced on the cytosolic side of the plasma membrane in response to certain signals, as we discuss later. Together, these interaction domains allow signaling proteins to bind to the membrane and to one another in specific combinations, forming three-dimensional networks that establish the route each signaling pathway will take. Because interaction domains tend to be located within flexible, unstructured regions of the protein—and proteins can contain multiple interaction domains—activation of a receptor can nucleate the formation of a large, gel-like matrix of cross-linked proteins at the plasma membrane (Figure 16–30). These sprawling aggregates act as specialized subcompartments, called biomolecular condensates, which are held together by numerous weak interactions, as we discuss in Chapter 4 (see Figure 4–52).
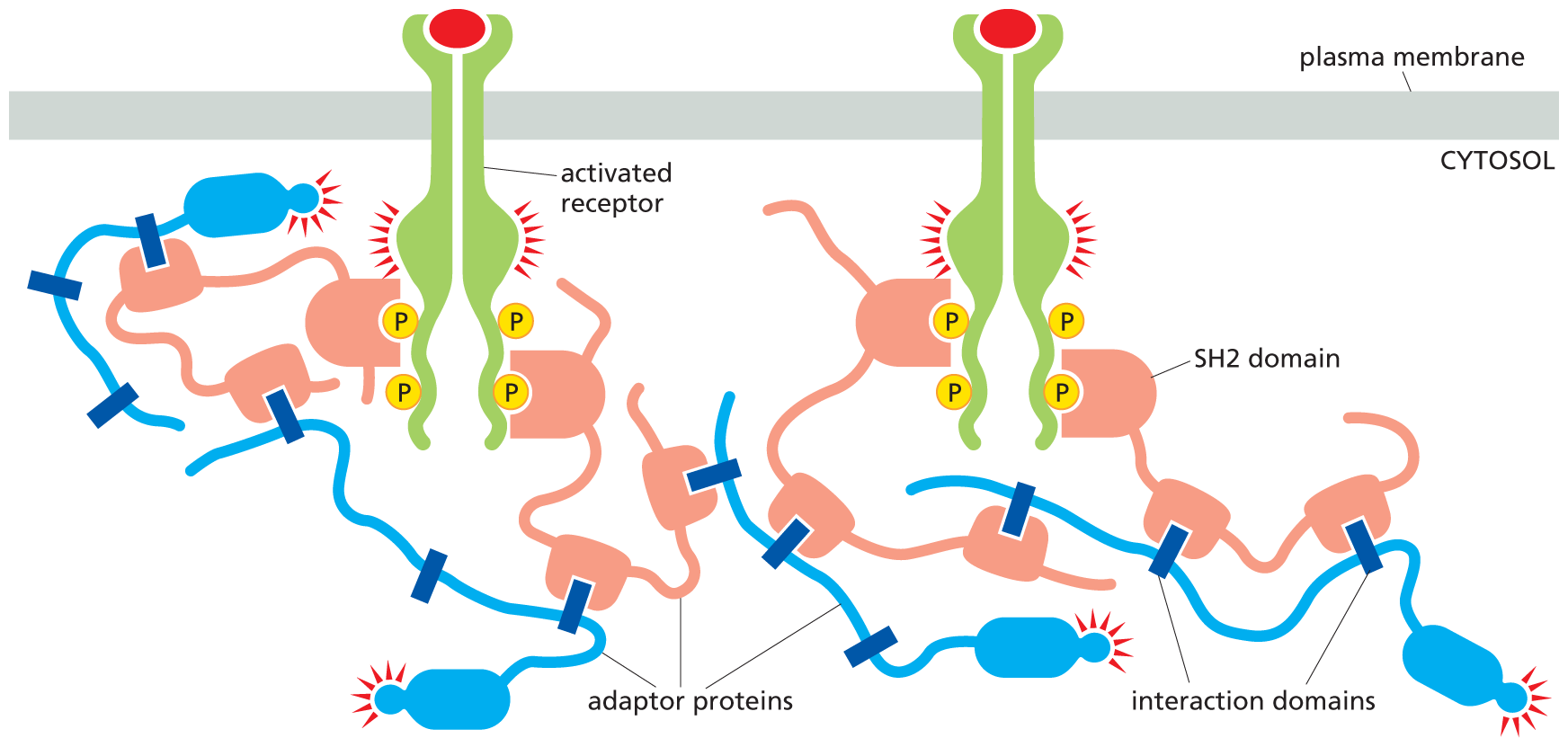
An illustration shows intracellular signaling proteins creating a cross-linked matrix at the plasma membrane. Two activated receptors bound to the plasma membrane are shown. Phosphates on the cytosol side of the receptors link them to S H 2 domains of adaptor proteins. The S H 2 domains are bound to proline-rich interaction domains of multiple different adaptor proteins, creating a matrix structure.
Figure 16–30 Intracellular signaling proteins interact to form a cross-linked, gel-like matrix at the plasma membrane. Adaptor proteins contain multiple interaction domains located within flexible, unstructured regions; some (such as SH2 domains) bind to phosphorylated tyrosines on the tails of activated RTKs, while others recognize the proline-rich interaction domains (dark blue) of different adaptor proteins (blue). These multivalent interactions drive the assembly of complex, cross-linked, three-dimensional signaling networks. Some adaptors contain protein kinase domains that become activated within the complex, thereby strengthening and relaying the signal onward. Such aggregates are examples of membraneless subcompartments or biomolecular condensates that are held together by weak noncovalent interactions (see Figure 4–52).
As long as they remain together, the signaling protein complexes assembled on the cytosolic tails of the RTKs can transmit a signal along several routes simultaneously to many destinations in the cell, thus activating and coordinating the numerous biochemical changes that are required to trigger a complex response such as cell proliferation or differentiation. To help terminate the response, the tyrosine phosphorylations are reversed by tyrosine phosphatases, which remove the phosphates that were added to the tyrosines of both the RTKs and other intracellular signaling proteins in response to the extracellular signal. In some cases, activated RTKs (like some GPCRs) are inactivated in a more brutal way: they are dragged into the interior of the cell by endocytosis and then destroyed by digestion in lysosomes (as discussed in Chapter 15).
Different RTKs recruit different collections of intracellular signaling proteins, producing different effects; however, certain components are used by most RTKs. These include, for example, a phospholipase C that functions in the same way as the phospholipase C activated by GPCRs to trigger the inositol phospholipid signaling pathway discussed earlier (see Figure 16–23). Another intracellular signaling protein that is activated by almost all RTKs is a small GTP-binding protein called Ras, as we discuss next.
As we have seen, activated RTKs recruit and activate many kinds of intracellular signaling proteins, leading to the formation of large signaling complexes on the cytosolic tail of the RTK. One of the key members of these signaling complexes is Ras—a small GTP-binding protein that is attached by a lipid tail to the cytosolic face of the plasma membrane. Virtually all RTKs activate Ras, including platelet-derived growth factor (PDGF) receptors, which mediate cell proliferation in wound healing, and nerve growth factor (NGF) receptors, which play an important part in the development of certain vertebrate neurons.
The Ras protein is a member of a large family of small GTP-binding proteins, often called monomeric GTPases to distinguish them from the trimeric G proteins that we encountered earlier. Ras resembles the α subunit of a G protein and functions as a molecular switch in much the same way. It cycles between two distinct conformational states—active when GTP is bound and inactive when GDP is bound. Interaction with an activating protein called Ras GEF encourages Ras to exchange its GDP for GTP, thus switching Ras to its activated state (Figure 16–31); after a delay, Ras is switched off by a GAP called Ras GAP (see Figure 16–12), which promotes the hydrolysis of its bound GTP to GDP (Movie 16.7).
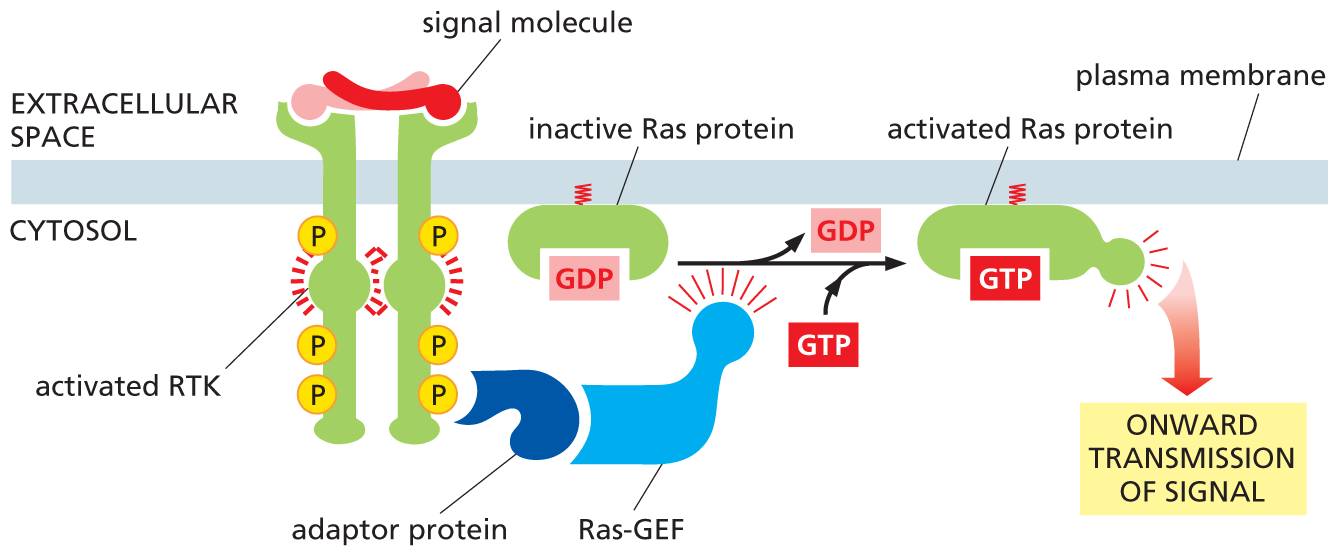
An illustration depicts the formation of an activated Ras protein from an activated R T K. An extracellular signal molecule binds with R T K and activates it bringing two R T K together. The activated R T K had phosphates on the ccytosol side. An adaptor protein bound to Ras G E F interacts with a phosphate on the R t K. This mediates the conversion of inactive Ras protein with G D P to an activated Ras protein with G T P. In the process, G D P is released and G T P is added. The activated Ras protein promotes the onward transmission of signal.
Figure 16–31 RTKs activate Ras. An adaptor protein docks on a particular phosphotyrosine on the activated receptor (the other signaling proteins that would be bound to the receptor, as shown in Figure 16–29, have been omitted for simplicity). The adaptor recruits a Ras guanine nucleotide exchange factor (Ras GEF) that stimulates Ras to exchange its bound GDP for GTP. The activated Ras protein can now stimulate several downstream signaling pathways, one of which is shown in Figure 16–32. Note that the Ras protein contains a covalently attached lipid group (red) that helps anchor the protein to the inside of the plasma membrane.
In its active state, Ras initiates a phosphorylation cascade in which a series of serine/threonine kinases phosphorylate and activate one another in sequence, like an intracellular game of dominoes. This relay system, which carries the signal from the plasma membrane to the nucleus, includes a three-kinase module called the MAP-kinase signaling module, in honor of the final enzyme in the chain, the mitogen-activated protein kinase, or MAP kinase. (As we discuss in Chapter 18, mitogens are extracellular signal molecules that stimulate cell proliferation.) In this pathway, outlined in Figure 16–32, MAP kinase is phosphorylated and activated by an enzyme called, logically enough, MAP kinase kinase. This protein is itself switched on by a MAP kinase kinase kinase (which is activated by Ras). At the end of the MAP-kinase cascade, MAP kinase phosphorylates various effector proteins, including certain transcription regulators, altering their ability to control gene transcription. The resulting change in the pattern of gene expression may stimulate cell proliferation, promote cell survival, or induce cell differentiation: the precise outcome will depend on which other genes are active in the cell and what other signals the cell receives. How biologists unravel such complex signaling pathways is discussed in How We Know, pp. 584–585.
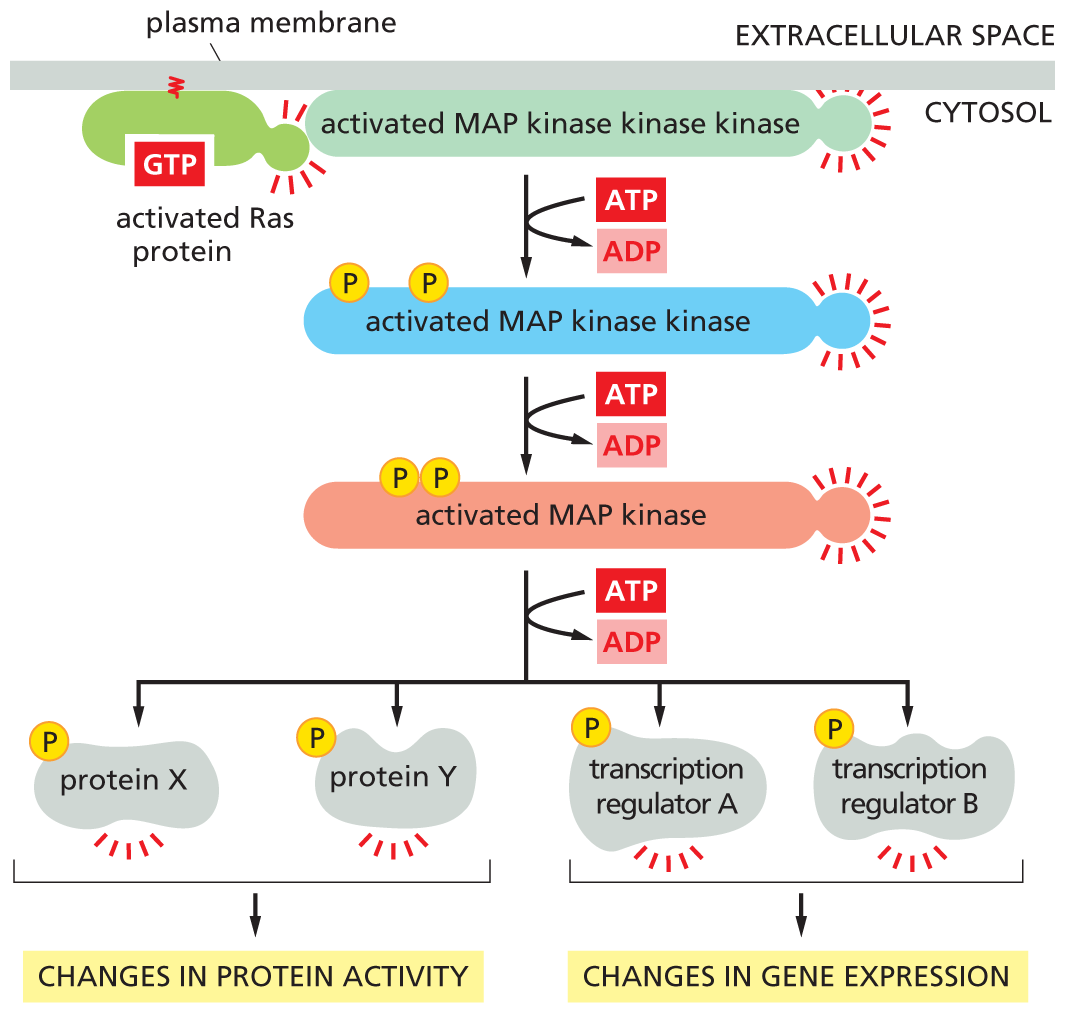
A flow diagram shows how Ras activates the signaling module of M A P kinase. An activated Ras protein activates a MAP kinase kinase kinase in the cytosol. A T P is converted to A D P which leads to an activated M A P kinase kinase with two phosphates. The activated M A P kinase kinase forms an activated M A P kinase by conversion of A T P to A D P. Activated M A P kinase forms activated protein X, activated protein Y, activated transcription regulator A, and activated transcription regulator B through the conversion of A T P to A D P. Protein X and Protein Y are responsible for changes in protein activity. Transcription regulator A and transcription regulator B are responsible for changes in gene expression.
Figure 16–32 Ras activates a MAP-kinase signaling module. The Ras protein, activated by the process shown in Figure 16–31, activates a three-kinase signaling module, which relays the signal onward. The final kinase in the module, MAP kinase, phosphorylates various downstream signaling or effector proteins.
Before Ras was discovered in normal cells, a mutant form of the protein was found in human cancer cells. The mutation inactivates the GTPase activity of Ras, so that the protein cannot shut itself off, promoting uncontrolled cell proliferation and the development of cancer. About 30% of human cancers contain such activating mutations in a Ras gene; of the cancers that do not, many have mutations in genes that encode proteins that function in the same signaling pathway as Ras. Many of the genes that encode normal intracellular signaling proteins were initially identified in the hunt for cancer-promoting oncogenes (discussed in Chapter 20).
QUESTION 16–7
Would you expect to activate RTKs by exposing the exterior of cells to antibodies that bind to the respective proteins? Would your answer be different for GPCRs? (Hint: Review Panel 4–2, on pp. 144–145, regarding the properties of antibody molecules.)
Many of the extracellular signal proteins that stimulate animal cells to survive and grow, including signal proteins belonging to the insulin-like growth factor (IGF) family, act through RTKs. One crucially important signaling pathway that these RTKs activate to promote cell growth and survival involves the enzyme phosphoinositide 3-kinase (PI 3-kinase), which phosphorylates inositol phospholipids in the plasma membrane. These phosphorylated lipids serve as docking sites for specific intracellular signaling proteins, which relocate from the cytosol to the plasma membrane, where they can activate one another. One of the most important of these relocated signaling proteins is the serine/threonine kinase Akt (Figure 16–33).
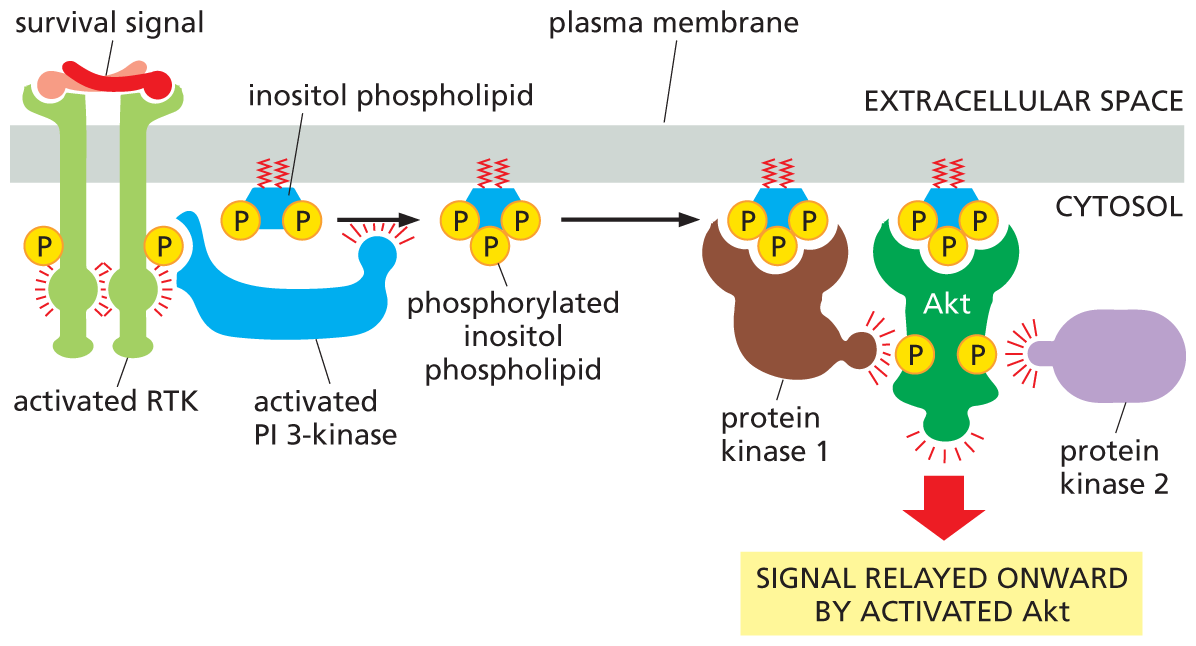
An illustration shows how R T K activates the P I 3 kinase-A K T signaling pathway. An extracellular survival signal molecule binds with an R T K and activates it. The activated R T K activates P I 3 kinase, which aids the conversion of inositol phospholipid to phosphorylated inositol phospholipid. Phosphorylated inositol phospholipid activates protein kinase 1. Activated protein kinase 1 along with activated protein kinase 2 from the cytosol work together to activate A K T, causing it to relay the signal onward.
Figure 16–33 Some RTKs activate the PI-3-kinase–Akt signaling pathway. An extracellular survival signal, such as IGF, activates an RTK, which recruits and activates PI 3-kinase. PI 3-kinase then phosphorylates an inositol phospholipid that is embedded in the cytosolic side of the plasma membrane. The resulting phosphorylated inositol phospholipid attracts intracellular signaling proteins that have an interaction domain that recognizes it. One of these signaling proteins, Akt, is a protein kinase that is activated at the membrane by phosphorylation mediated by two other protein kinases (here called protein kinases 1 and 2); protein kinase 1 is also recruited by the phosphorylated lipid docking sites. Once activated, Akt is released from the plasma membrane and phosphorylates various downstream proteins on specific serines and threonines (not shown).
Akt, also called protein kinase B (PKB), promotes the growth and survival of many cell types, often by inactivating the signaling proteins it phosphorylates. For example, Akt phosphorylates and inactivates a cytosolic protein called Bad. In its active state, Bad encourages the cell to kill itself by indirectly activating a cell-suicide program called apoptosis (discussed in Chapter 18). Phosphorylation by Akt thus promotes cell survival by inactivating a protein that otherwise promotes cell death (Figure 16–34).
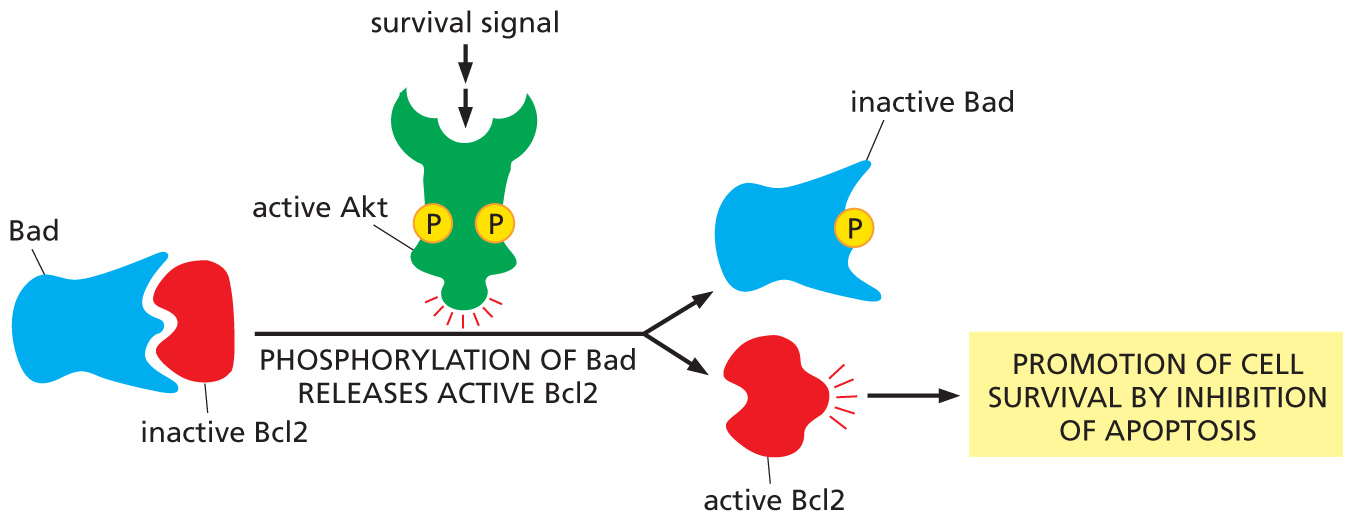
An illustration shows how an activated A K T promotes cell survival. A Bad protein binds with an inactive B C l 2 in the first step. A survival signal activates A k t, and phosphorylation of the Bad protein takes place. This releases active B C l 2 and inactive bad protein. As a result, active B C I 2 leads to the promotion of cell survival by inhibition of apoptosis.
Figure 16–34 Activation of Akt promotes cell survival. One way Akt promotes cell survival is by phosphorylating and inactivating a protein called Bad. In its unphosphorylated state, Bad promotes apoptosis (a form of cell death) by binding to and inhibiting a protein, called Bcl2, which otherwise suppresses apoptosis. When Bad is phosphorylated by Akt, Bad releases Bcl2, which now blocks apoptosis, thereby promoting cell survival.
In addition to promoting cell survival, the PI-3-kinase–Akt signaling pathway stimulates cells to grow in size. It does so by indirectly activating a large serine/threonine kinase called Tor. Tor stimulates cells to grow both by enhancing protein synthesis and by inhibiting protein degradation (Figure 16–35). The anticancer drug rapamycin works by inactivating Tor, indicating the importance of this signaling pathway in regulating cell growth and survival—and the consequences of its dysregulation in cancer.
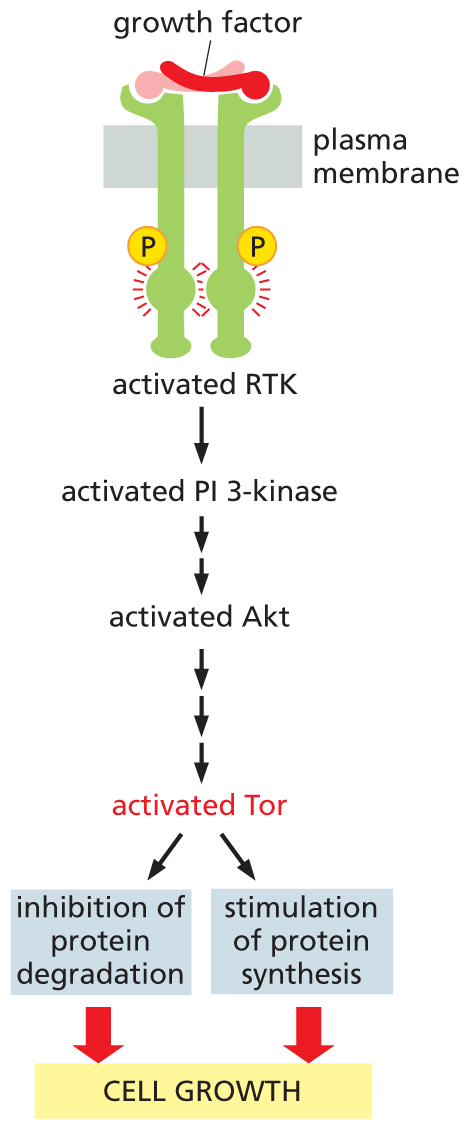
A flow diagram shows how A K T stimulates cell growth. A growth factor signal molecule binds to an R T K bound to two phosphates, and activates it. Activated R T K leads to activated P I 3 kinase, which leads to activated A k t. The activated A k t leads to activated T o r, causing the inhibition of protein degradation and stimulation of protein synthesis, both of which promote cell growth.
Figure 16–35 Akt stimulates cells to grow in size by activating the serine/threonine kinase Tor. The binding of a growth factor to an RTK activates the PI-3-kinase–Akt signaling pathway (as shown in Figure 16–33). Akt then indirectly activates Tor by phosphorylating and inhibiting a protein that helps to keep Tor shut down (not shown). Tor stimulates protein synthesis and inhibits protein degradation by phosphorylating key proteins in these processes (not shown). The anticancer drug rapamycin slows cell growth by inhibiting Tor. In fact, the Tor protein derives its name from the fact that it is a target of rapamycin.
The main intracellular signaling cascades activated by GPCRs and RTKs are summarized in Figure 16–36. As dauntingly complex as such pathways may seem, the complexity of cell signaling is actually much greater still. First, although we depict these signaling pathways as being relatively linear and self-contained, they do not operate entirely independently and engage in a great deal of cross-talk. Second, we have described only a very small handful of the intracellular signaling pathways that operate in cells. So before we return to a discussion of signal integration, we take a brief detour to introduce a few additional types of signaling systems that we have thus far overlooked.
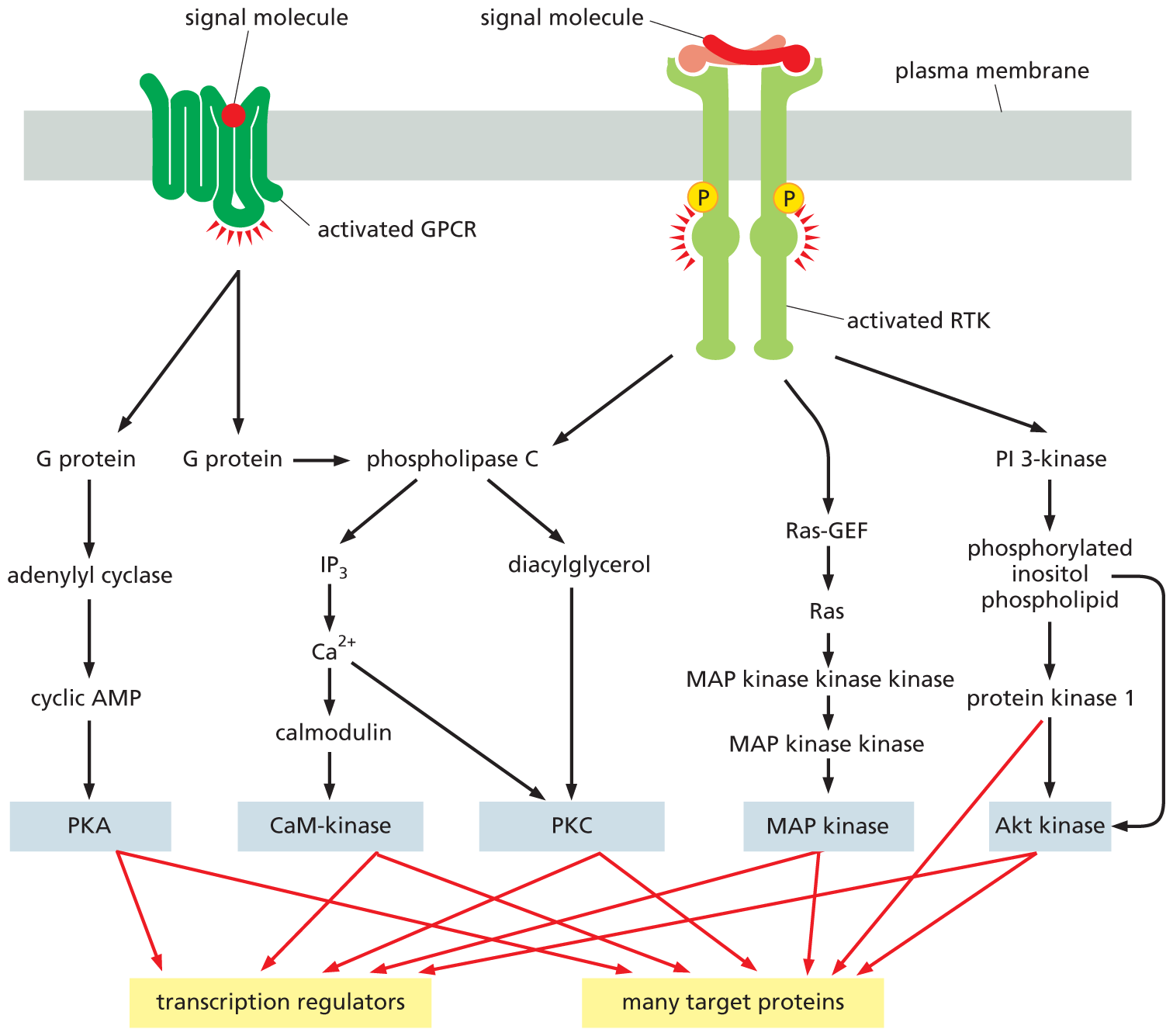
An illustration shows the multiple intracellular signaling pathways activated by G P C R and R T K. A signal molecule binds with G P C R and activates it. Activated G P C R forms two G proteins, of which one activates phospholipase C. The other G protein forms adenylyl cyclase which forms cyclic A M P. Cyclic A M P forms P K A, which then activates transcription regulators and many target proteins. Phospholipase C forms I P subscript 3 and diacylglycerol. I P subscript 3 forms C a 2 plus ions, which then activates calmodulin. Calmodulin forms CaM-kinase, which forms transcription regulators and many target proteins. Diacylglycerol activated by phospholipase C activates P K C, which activates transcription regulators and many target proteins. On the other side of the diagram, a signal molecule binds with R T K and activates it. Activated R T K activates phospholipase C, Ras-G E F, and P I 3-kinase. Ras G E F forms Ras, then M A P kinase kinase kinase. M A P kinase kinase kinase forms M A P kinase kinase, then M A P kinase, which activates transcription regulators and many target proteins. P I 3-kinase activates phosphorylated inositol phospholipid which activates protein kinase 1 and A k t kinase. Protein kinase 1 also activates A k t kinase, which then forms transcription regulators and many target proteins.
Figure 16–36 Both GPCRs and RTKs activate multiple intracellular signaling pathways. The figure reviews five of these pathways: two leading from GPCRs—through adenylyl cyclase and through phospholipase C—and three leading from RTKs—through phospholipase C, Ras, and PI 3-kinase. Each pathway differs from the others, yet they use some common components to transmit their signals. Because all five eventually activate protein kinases (gray boxes), it seems that each is capable in principle of regulating practically any process in the cell.
Intracellular signaling pathways are never mapped out in a single experiment. Although insulin was first isolated from dog pancreas in the early 1920s, the molecular chain of events that links the binding of insulin to its receptor with the activation of the transporter proteins that take up glucose has taken decades to untangle—and is still not completely understood.
Instead, investigators figure out, piece by piece, how all the links in the chain fit together—and how each contributes to the cell’s response to an extracellular signal molecule such as the hormone insulin. Here, we discuss the kinds of experiments that allow scientists to identify individual links and, ultimately, to piece together complex signaling pathways.
Most signaling pathways depend on proteins that physically interact with one another. There are several ways to detect such direct contact. One involves using a protein as “bait.” For example, to isolate the receptor that binds to insulin, one could attach insulin to a chromatography column. Cells that respond to the hormone are broken open with detergents that disrupt their membranes, releasing the transmembrane receptor proteins (see Figure 11–27). When this slurry is poured over the chromatography column, the proteins that bind to insulin will stick to the column and can later be eluted and identified (see Figure 4–54).
Protein–protein interactions in a signaling pathway can also be identified by co-immunoprecipitation. For example, cells exposed to an extracellular signal molecule can be broken open, and antibodies can be used to grab the receptor protein known to recognize the signal molecule (see Panel 4–2, pp. 144–145). If the receptor is strongly associated with other proteins, as shown in Figure 16–29, these will be captured as well. In this way, researchers can identify which proteins interact when an extracellular signal molecule stimulates cells.
Once two proteins are known to bind to each other, an investigator can pinpoint which parts of the proteins are required for the interaction using the DNA technology discussed in Chapter 10. For example, to determine which phosphorylated tyrosine on a receptor tyrosine kinase (RTK) is recognized by a certain intracellular signaling protein, a series of mutant receptors can be constructed, each missing a different tyrosine from its cytoplasmic domain (Figure 16–37). In this way, the specific tyrosines required for binding can be determined. Similarly, one can determine whether this phosphotyrosine docking site is required for the receptor to transmit a signal to the cell.
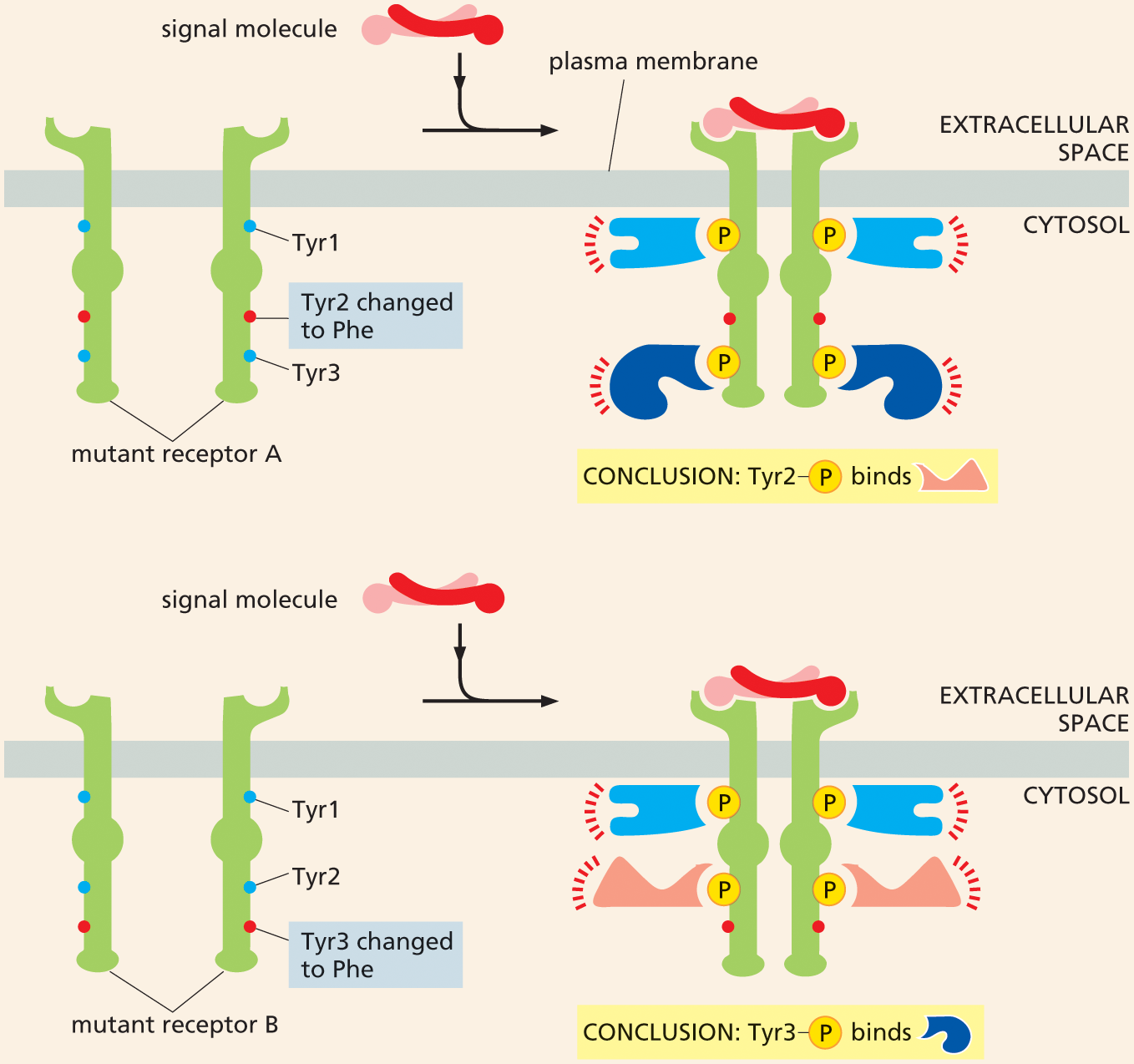
Two illustrations depict the role of mutant proteins in determining where an intracellular signaling molecule binds. The first part shows a mutant receptor A attached to the plasma membrane. Mutant receptor A has T Y R 1, T Y R 2 changed to P H E (phenylalanine), and T Y R 3. An extracellular signal molecule binds with the receptor in the next step and activates it. The two units of mutant receptor A associate to form four phosphorylated tyrosines in the cytosol. These tyrosines bind different intracellular signaling proteins, thus activating them and passing on the signal. The T Y R 2 changed to P H E (phenylalanine) is not shown to be bound to a phosphate or intracellular signaling protein.
The second part shows mutant receptor B with a similar configuration, with T Y R 1, T Y R 2, and T Y R 3 is changed to P h e. A signal molecule binds with the receptor and activates it. The two units of mutant receptor B associate to form four phosphorylated tyrosines in the cytosol. These tyrosines bind different intracellular signaling proteins, thus activating them and passing on the signal. The T Y R 3 changed to P H E (phenylalanine) is not shown to be bound to a phosphate or intracellular signaling protein.
Figure 16–37 Mutant proteins can help to determine exactly where an intracellular signaling molecule binds. As shown in Figure 16–29, on binding their extracellular signal molecule, a pair of RTKs come together and phosphorylate specific tyrosines on each other’s cytoplasmic tails. These phosphorylated tyrosines bind different intracellular signaling proteins, which then become activated and pass on the signal. To determine which tyrosine binds to a specific intracellular signaling protein, a series of mutant receptors is constructed. In the mutants shown, tyrosines Tyr2 or Tyr3 have been replaced, one at a time, by phenylalanine (red), thereby preventing phosphorylation at that site. As a result, the mutant receptors no longer bind to one of the intracellular signaling proteins shown in Figure 16–29. The effect on the cell’s response to the signal can then be determined. It is important that the mutant receptor be tested in a cell that does not have its own normal receptors for the signal molecule.
Ultimately, one wants to assess what role a particular protein plays in a signaling pathway. A first test may involve using DNA technology to introduce into cells a gene encoding a constantly active form of the protein, to see if this mimics the effect of the extracellular signal molecule. Consider Ras, for example. The mutant form of Ras involved in human cancers is constantly active because it has lost its ability to hydrolyze the bound GTP that keeps the Ras protein switched on. This continuously active form of Ras can stimulate some cells to proliferate, even in the absence of a proliferation signal.
Conversely, the activity of a specific signaling protein can be inhibited or eliminated. In the case of Ras, for example, one could shut down the expression of the Ras gene in cells by RNA interference or using the gene-editing tool CRISPR (see Figure 10–33). Such cells do not proliferate in response to extracellular mitogens, indicating the importance of normal Ras signaling in the proliferative response.
Another powerful strategy that scientists use to determine which proteins participate in cell signaling involves screening tens of thousands of animals—fruit flies or nematode worms, for example (discussed in Chapter 19)—to search for mutants in which a signaling pathway is not functioning properly. By examining enough mutant animals, many of the genes that encode the proteins involved in a signaling pathway can be identified.
Such classical genetic screens can also help sort out the order in which intracellular signaling proteins act in a pathway. Suppose that a genetic screen uncovers a pair of new proteins, X and Y, involved in the Ras signaling pathway. To determine whether these proteins lie upstream or downstream of Ras, one could create cells that express an inactive, mutant form of each protein, and then ask whether these mutant cells can be “rescued” by the addition of a continuously active form of Ras. If the constantly active Ras overcomes the blockage created by the mutant protein, the protein must operate upstream of Ras in the pathway (Figure 16–38A). However, if Ras operates upstream of the protein, a constantly active Ras would be unable to transmit a signal past the obstruction caused by the disabled protein (Figure 16–38B). Through such experiments, even the most complex intracellular signaling pathways can be mapped out, one step at a time (Figure 16–38C).
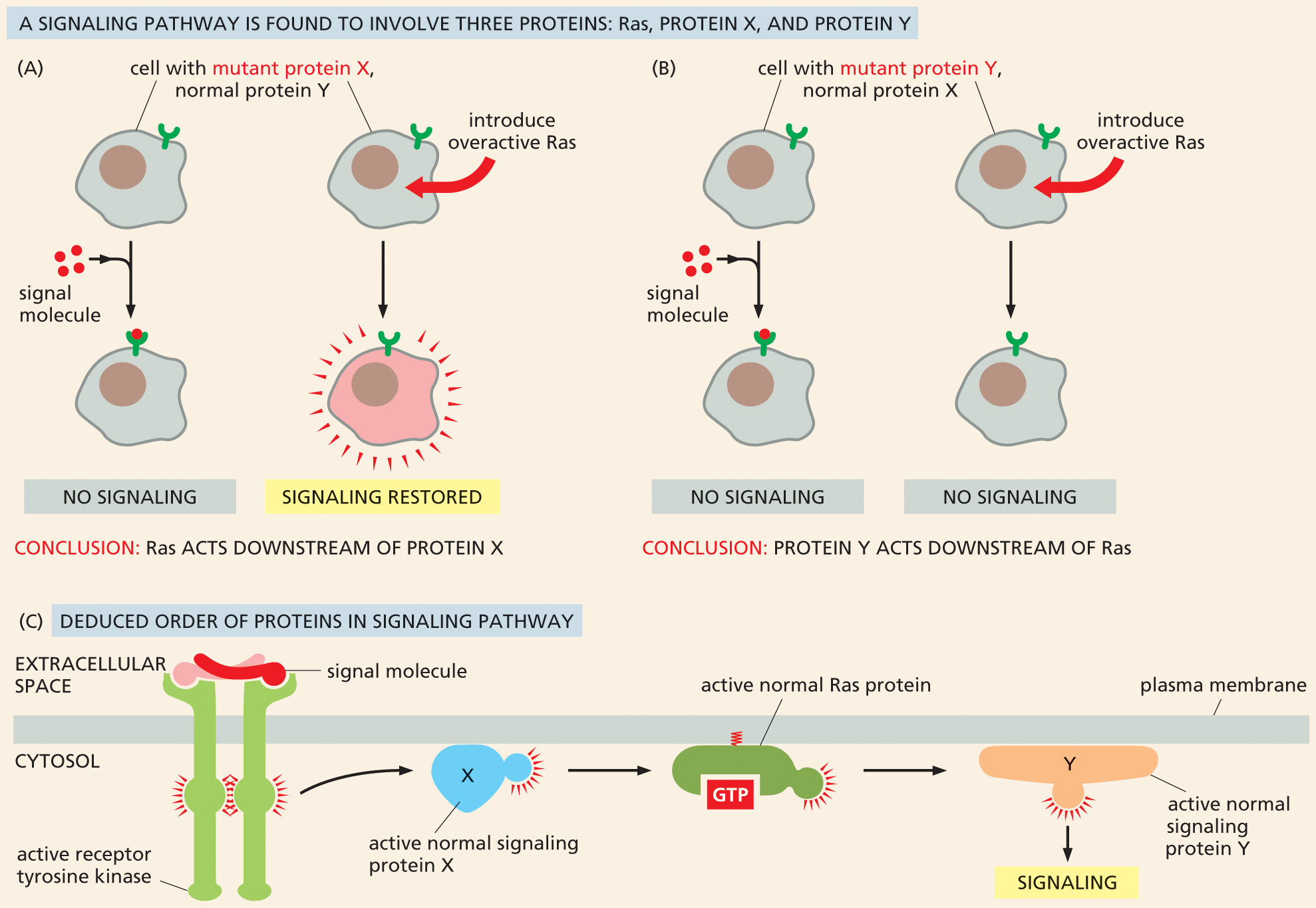
Illustration A shows Ras acting downstream of protein X in a signaling pathway. Text corresponding to illustration A and B reads, a signaling pathway is found to involve three proteins; Ras, protein X and protein Y. Illustration A depicts two cells with mutant protein X and normal protein Y, each with a receptor protein. Signal molecules bind to the receptor in one cell, and no signaling takes place.
When overactive Ras is introduced to the other cell, signaling is restored. Conclusion reads: Ras acts downstream of protein X.
Illustration B shows protein Y acting downstream of Ras in a signaling pathway. Illustration B depicts two cells with mutant protein Y and normal protein X, each with a receptor protein. Signal molecules bind to the receptor in one of the cells, and no signaling takes place. Overactive Ras is introduced in the other cell, and no signaling takes place. Conclusion reads: Protein Y acts downstream of Ras.
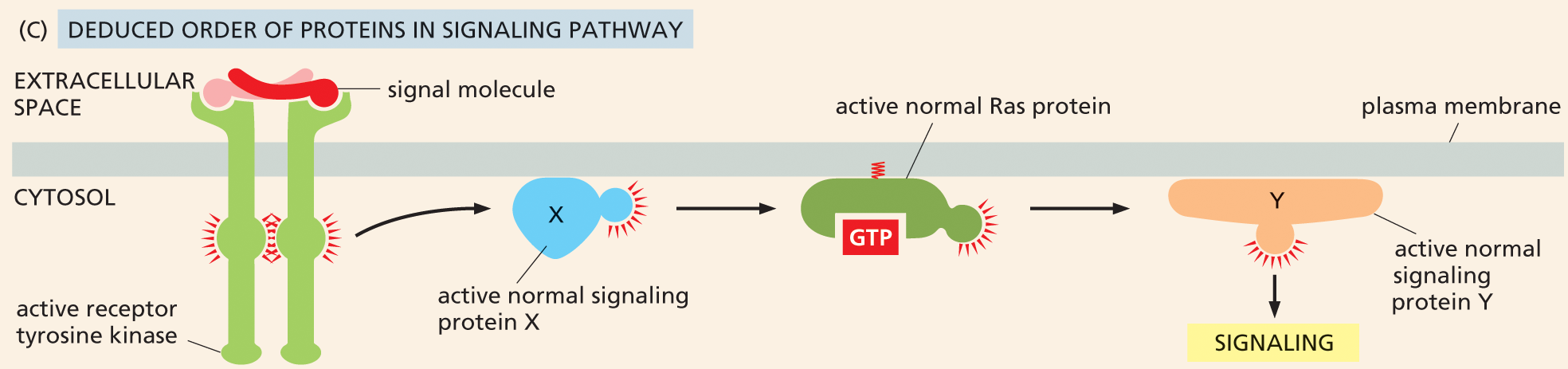
Illustration C shows how the order of the signaling pathway is deduced. The deduced order of the signaling pathway is shown, beginning with an active receptor tyrosine kinase spanning the plasma membrane bound to an extracellular signal molecule. This leads to an active normal signaling protein X, which then leads to an active normal Ras protein bound to G T P. Finally, this leads to an active normal signaling protein Y, which causes signaling.
Figure 16–38 The use of mutant cell lines and an overactive form of Ras can help dissect an intracellular signaling pathway. In this hypothetical pathway, Ras, protein X, and protein Y are required for proper signaling. (A) In cells in which protein X has been inactivated, signaling does not occur. However, this signaling blockage can be overcome by the addition of an overactive form of Ras, such that the pathway is active even in the absence of the extracellular signal molecule. This result indicates that Ras acts downstream of protein X in the pathway. (B) Signaling is also disrupted in cells in which protein Y has been inactivated. In this case, introduction of an overactive Ras does not restore normal signaling, indicating that protein Y operates downstream of Ras. (C) Based on these results, the deduced order of the signaling pathway is shown.
Not all receptors trigger complex signaling cascades that use multiple components to carry a message to the nucleus. Some take a more direct route to control gene expression. One such receptor is the protein Notch. Notch is a crucially important receptor in all animals, both during development and in adults. Among other things, it controls the development of neural cells in Drosophila (Figure 16–39).
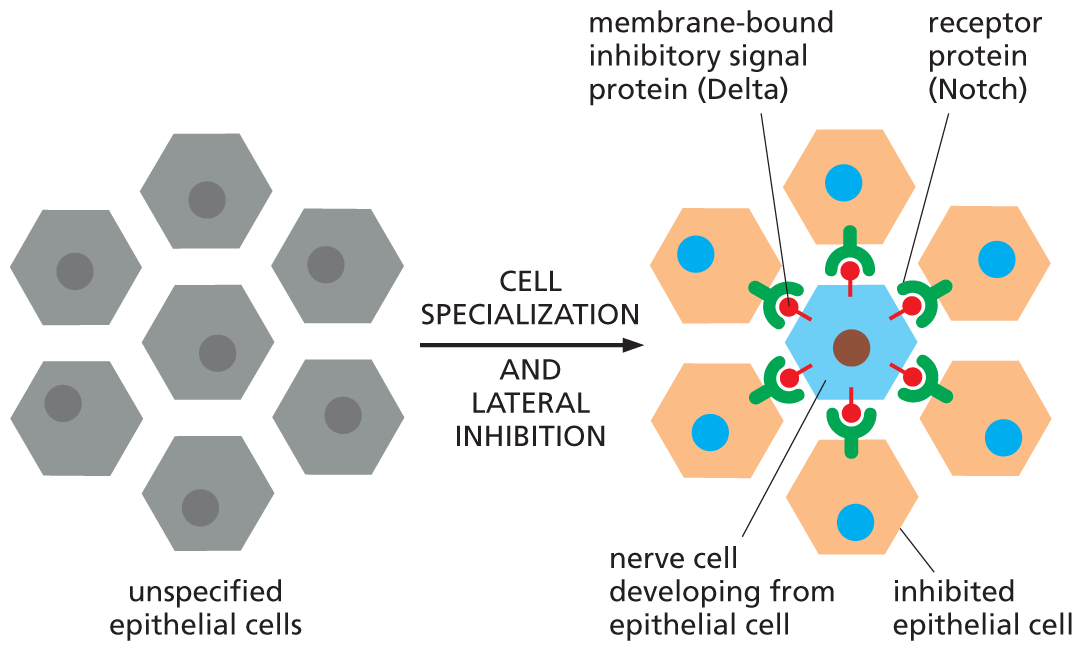
An illustration shows nerve cell production in Drosophila using notch signaling. A cluster of unspecified epithelial cells are shown. These cells undergo cell specialization and lateral inhibition to form different components which are labeled as follows: membrane bound inhibitory signal protein (Delta), receptor protein (notch), nerve cell developing from epithelial cell, and inhibited epithelial cell. The nerve cell is at the center. The nerve cell has signal proteins attached to it, which are bound to receptor proteins of inhibited epithelial cells surrounding the nerve cell.
Figure 16–39 Notch signaling controls nerve-cell production in the fruit fly Drosophila. The fly nervous system originates in the embryo from a sheet of epithelial cells. Isolated cells in this sheet begin to specialize as neurons (blue), while their neighbors remain non-neuronal and maintain the structure of the epithelial sheet. The signals that control this process are transmitted via direct cell–cell contacts: each future neuron delivers an inhibitory signal to the cells next to it, deterring them from specializing as neurons, too—a process called lateral inhibition. Both the signal molecule (Delta) and the receptor molecule (Notch) are transmembrane proteins, and the pathway represents a form of contact-dependent signaling (see Figure 16–3D).
In this simple signaling pathway, the receptor itself acts as a transcription regulator. When activated by the binding of Delta, a transmembrane signal protein on the surface of a neighboring cell, the Notch receptor is cleaved. This cleavage releases the cytosolic tail of the receptor, which is then free to move to the nucleus, where it helps to activate the appropriate set of Notch-responsive genes (Figure 16–40).
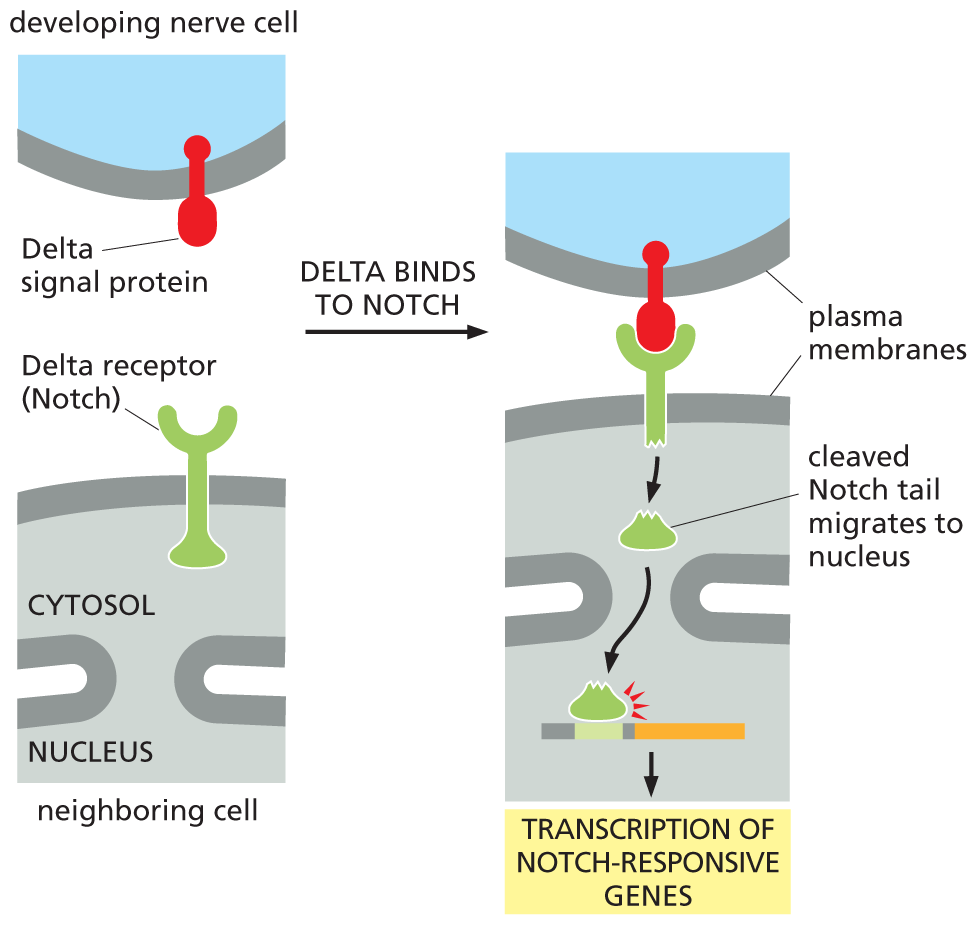
An illustration depicts transcription of the notch responsive gene. A developing nerve cell with a delta signal protein bound to the plasma membrane is shown on top, and a neighboring cell with a delta receptor (notch) on its plasma membrane is shown below it. Delta binds to notch in the next step and the tail of the delta receptor inside the cell is cleaved. The cleaved notch tail migrates to nucleus causing the transcription of notch-responsive genes.
Figure 16–40 The Notch receptor itself is a transcription regulator. When the membrane-bound signal protein Delta binds to its receptor, Notch, on a neighboring cell, the receptor is cleaved by a protease. The released part of the cytosolic tail of Notch migrates to the nucleus, where it activates Notch-responsive genes. One consequence of this signaling process is shown in Figure 16–39.
Another direct route to the nucleus is taken by extracellular signal molecules that rely on intracellular receptor proteins (see Figure 16–4B). These molecules include the steroid hormones—cortisol, estradiol, and testosterone—and the thyroid hormones such as thyroxine (Figure 16–41). All of these hydrophobic molecules pass through the plasma membrane of the target cell and bind to receptor proteins located in either the cytosol or the nucleus. Regardless of their initial location, these intracellular receptor proteins are referred to as nuclear receptors because, when activated by hormone binding, they enter the nucleus, where they regulate the transcription of genes. In unstimulated cells, nuclear receptors are typically present in an inactive form. When a hormone binds, the receptor undergoes a large conformational change that activates the protein, allowing it to promote or inhibit the transcription of specific target genes (Figure 16–42). Each hormone binds to a different nuclear receptor, and each receptor acts at a different set of regulatory sites in DNA (discussed in Chapter 8). Moreover, a given hormone usually regulates different sets of genes in different cell types, thereby evoking different physiological responses in different target cells.
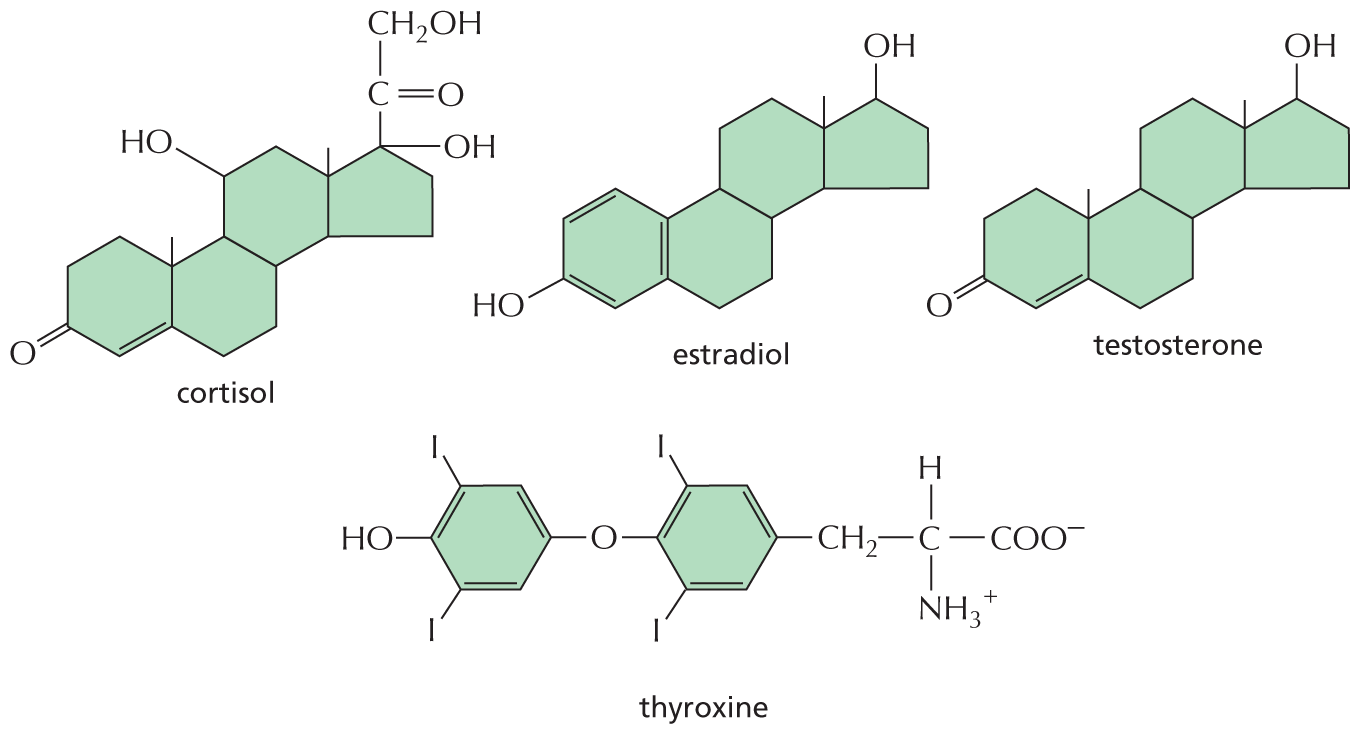
An illustration shows four chemical structures labeled cortisol, estradiol, testosterone, and thyroxine. Cortisol shows three six-membered rings fused to a five-membered ring. The topmost carbon of the five-membered ring is bonded to a hydroxyl group, another carbon is bonded to an oxygen atom and a hydroxymethyl group at the top. One of the 6-membered rings is a cyclohexenone ring. It is fused to second cyclohexane ring that is further fused to a cyclohexane at the top right. The carbon on the top left is bonded to a hydroxyl group.
The structure of Estradiol shows a phenol group bonded to a cyclohexane ring. This ring is further fused to another cyclohexane ring, fused further to a cyclopentane ring. The topmost carbon of the cyclopentane ring is bonded to a hydroxyl group. The structure of testosterone is similar to that of estradiol except that, the phenol ring is replaced by a cyclohexenone ring.
The structure of thyroxine shows two benzene rings. C 1 and C 3 of the first and the second benzene rings are bonded with Iodine and C 2 of the first benzene ring is bonded to a hydroxyl group. C 5 of the first benzene ring is bonded to oxygen atom which is further single bonded to C 2 of the second benzene ring. C 5 of the second benzene ring is bonded to two carbon groups in which the first carbon atom forms C H 2 and the second carbon atom is bonded to an ammonia cation, a hydrogen atom, and a carboxylate anion.
Figure 16–41 Some small, hydrophobic hormones bind to intracellular receptors that act as transcription regulators. Although these signal molecules differ in their chemical structures and functions, they all act by binding to intracellular receptor proteins that act as transcription regulators. Their receptors are not identical, but they are evolutionarily related, belonging to the nuclear receptor superfamily. The sites of origin and functions of these hormones are given in Table 16–1 (p. 556).
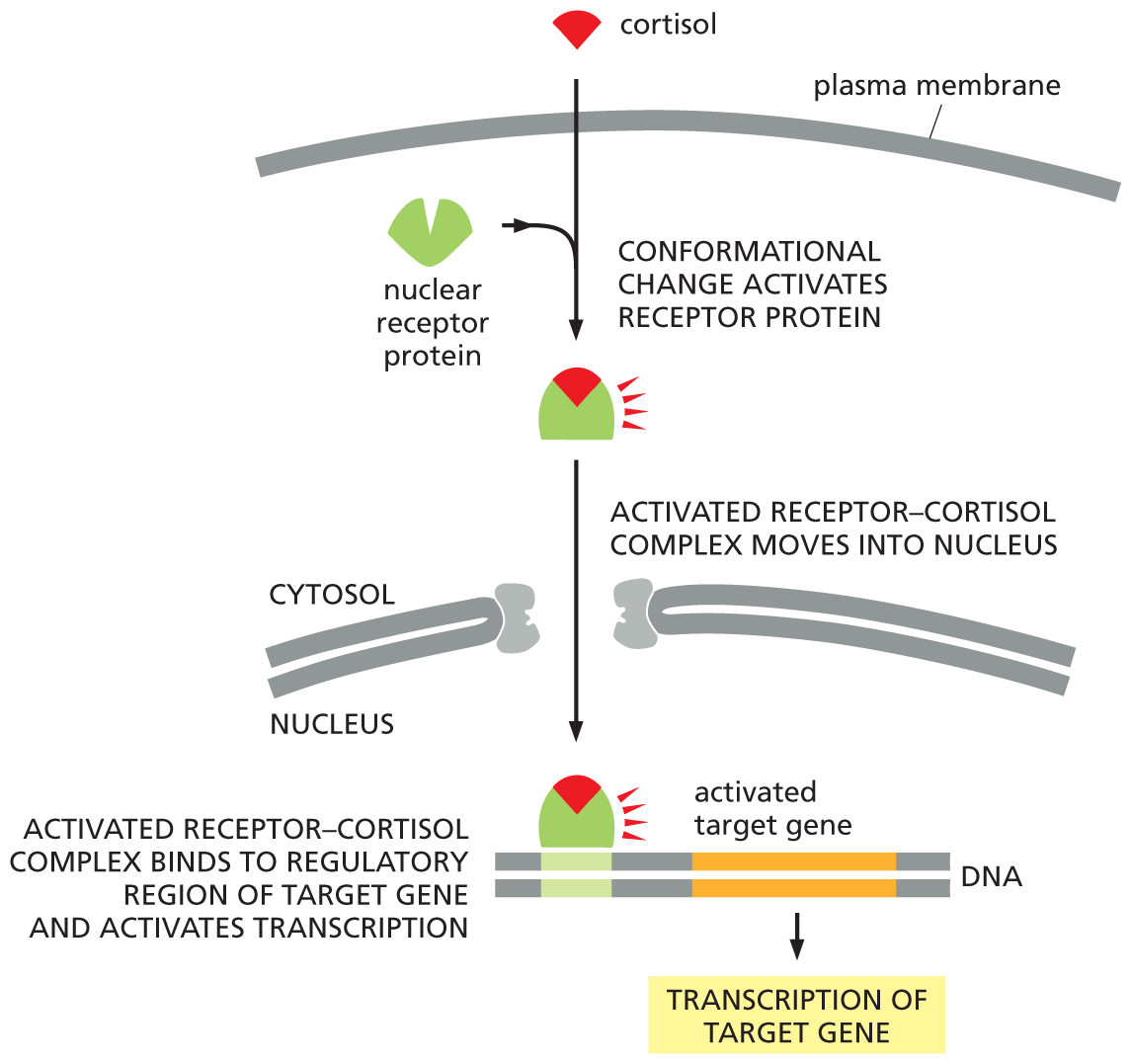
A flow diagram depicts how cortisol activates transcription of a target gene. Cortisol enters the cytosol through the plasma membrane and binds with a nuclear receptor protein in the first step. Text reads, conformational change activates receptor protein. The activated receptor-cortisol complex moves into the nucleus in the next step. Activated receptor-cortisol complex binds to regulatory region of target gene and activates transcription in the next step. A D N A strand is shown with the activated target gene labeled. The activated target gene leads to transcription of the target gene.
Figure 16–42 The steroid hormone cortisol activates a transcription regulator. Cortisol is one of the hormones produced by the adrenal glands in response to stress. This hydrophobic signal molecule crosses the plasma membrane of a target cell and binds to its receptor protein, which is located in the cytosol. The receptor–hormone complex is then transported into the nucleus via the nuclear pores. Cortisol binding activates the receptor protein, which is then able to bind to specific regulatory sequences in DNA and activate (or repress, not shown) the transcription of specific target genes. Whereas the receptors for cortisol and some other steroid hormones are located in the cytosol, those for other steroid hormones and for thyroid hormones are already bound to DNA in the nucleus even in the absence of hormone.
Nuclear receptors and the hormones that activate them have essential roles in human physiology (see Table 16–1, p. 556). Loss of these signaling systems can have dramatic consequences, as illustrated by the effects of mutations that eliminate the receptor for the male sex hormone testosterone. Testosterone in humans shapes the formation of the external genitalia and influences brain development in the fetus; at puberty, the hormone triggers the development of male secondary sexual characteristics. Some very rare individuals are genetically male—that is, they have both an X and a Y chromosome—but lack the testosterone receptor as a result of a mutation in the corresponding gene; thus, they make testosterone, but their cells cannot respond to it. As a result, these individuals develop as females, which is the path that sexual and brain development would take if no male or female hormones were produced. Such a sex reversal demonstrates the crucial role of the testosterone receptor in sexual development, and it also shows that the receptor is required not just in one cell type to mediate one effect of testosterone, but in many cell types to help produce the whole range of features that distinguish men from women.
Plants and animals have been evolving independently for more than a billion years, the last common ancestor being a single-celled eukaryote that most likely lived on its own. Because these kingdoms diverged so long ago, each has evolved its own molecular solutions to the complex problem of becoming multicellular. Thus the mechanisms for cell–cell communication in plants and animals are in some ways quite different. At the same time, however, plants and animals started with a common set of eukaryotic genes—including some used by single-celled organisms to communicate among themselves—so their signaling systems also show some similarities.
Like animals, plants make extensive use of transmembrane cell-surface receptors—especially enzyme-coupled receptors. The spindly mustard weed Arabidopsis thaliana, which serves as a model for understanding plant biology (see Figure 1–34), has hundreds of genes encoding receptor serine/threonine kinases. These are, however, structurally distinct from the receptor serine/threonine kinases found in animal cells (which we do not discuss in this chapter). The plant receptors are thought to play an important part in a large variety of cell signaling processes, including those governing plant growth, development, and disease resistance. In contrast to animal cells, plant cells seem not to use RTKs, steroid-hormone-type nuclear receptors, or cyclic AMP, and they seem to use few GPCRs.
One of the best-studied signaling systems in plants mediates the response of cells to ethylene—a gaseous hormone that regulates a diverse array of developmental processes, including seed germination and fruit ripening. Tomato growers use ethylene to ripen their fruit, even after it has been picked. Although ethylene receptors are not evolutionarily related to any of the classes of receptor proteins that we have discussed so far, they function as enzyme-coupled receptors. Surprisingly, it is the empty receptor that is active: in the absence of ethylene, the empty receptor activates an associated protein kinase that ultimately shuts off the ethylene-responsive genes in the nucleus; when ethylene is present, the receptor and kinase are inactive, and the ethylene-responsive genes are transcribed (Figure 16–43). This strategy, whereby signals act to relieve transcriptional inhibition, is commonly used in plants.
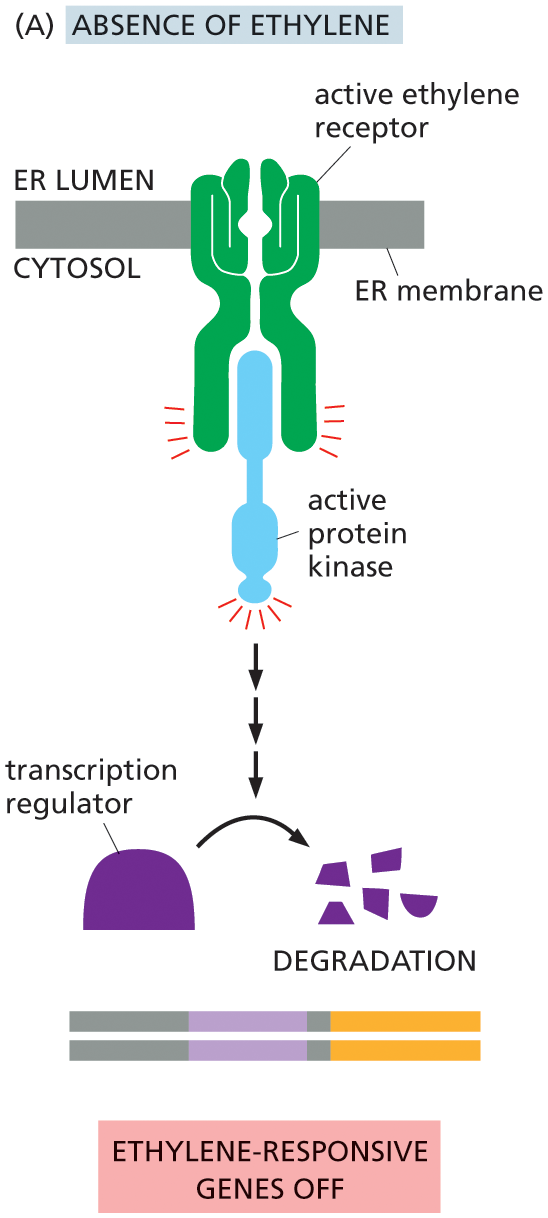
Illustration A depicts the ethylene signaling pathway in absence of ethylene. An active ethylene receptor protein is shown attached to the E R membrane. The active ethylene receptor activates protein kinase in the cytosol, which supports the degradation of transcription regulator, turning off the ethylene-responsive genes.
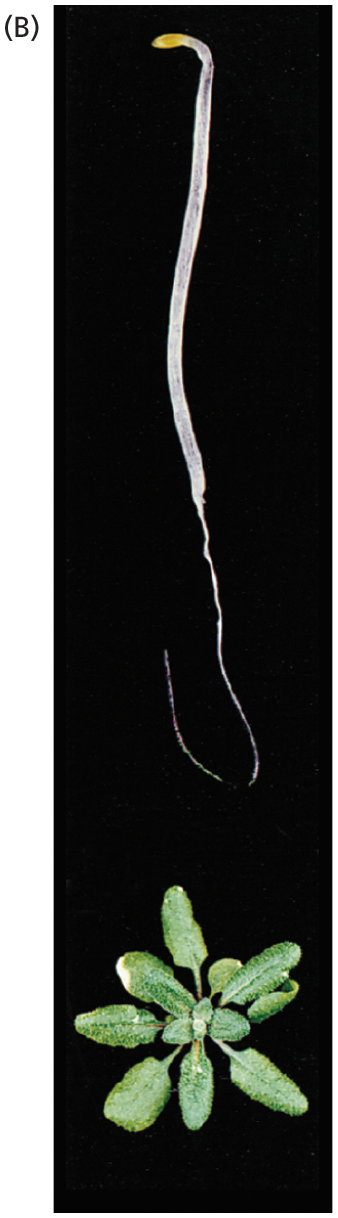
Image B shows a germinating plant seedling responding to the absence of ethylene. Above, the seedling stalk is shown growing straight upward, appearing long and thin. Below, the aboveground image of the plant is shown. It has a strong rosette of leaves.
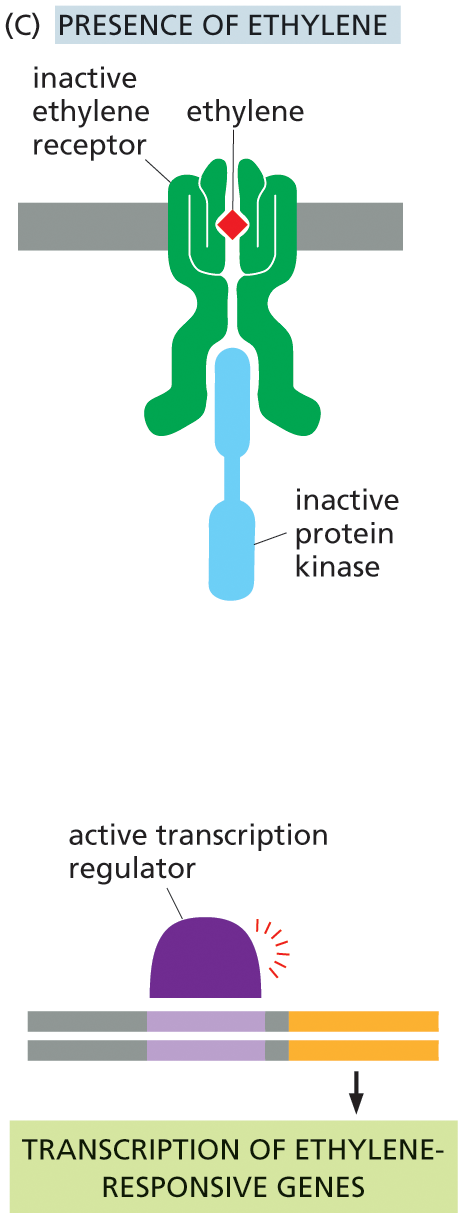
Illustration C depicts the ethylene signaling pathway in presence of ethylene. An ethylene molecule binds with an inactive ethylene receptor in the E R lumen. An inactive protein kinase binds with an inactive ethylene receptor on the cytosol side. An active transcription regulator leads to the transcription of ethylene responsive genes in the final step.

Image D shows a germinating plant seedling responding to the presence of ethylene. Above, the seedling stalk is shown growing short with a thick stem, and producing a more curved shape at the tip to protect the delicate tip to push obstacles out of the way. Below, the aboveground image of the plant is shown. It has limited leaf growth compared to image B, with smaller and fewer leaves.
Whether part of a plant or an animal, a cell receives messages from many sources, and it must integrate this information to generate an appropriate response: to live or die, to divide, to differentiate, to change shape, to move, to send out a chemical message of its own, and so on (see Figure 16–6, Movie 16.8, and Movie 16.9). This integration is made possible by connections and interactions that occur between different signaling pathways. Such cross-talk allows the cell to bring together multiple streams of information and react to a rich combination of signals.
The most extensive links among the pathways are mediated by the protein kinases present in each. These kinases often phosphorylate, and hence regulate, components in other signaling pathways, in addition to components in their own pathway (see Figure 16–36). To give an idea of the scale of the complexity of this web of potential interactions, genome sequencing studies suggest that about 2% of our 20,000 protein-coding genes code for protein kinases; moreover, hundreds of distinct types of protein kinases are thought to be present in a single mammalian cell.
Many intracellular signaling proteins have several potential phosphorylation sites, each of which can be phosphorylated by a different protein kinase. These proteins can thus act as integrating devices. Information received from different intracellular signaling pathways can converge on such proteins, which then convert a multicomponent input to a single outgoing signal (Figure 16–44, and see Figure 16–9). These integrating proteins, in turn, can deliver a signal to many downstream targets. In this way, the intracellular signaling system may act like a network of nerve cells in the brain—or like a collection of microprocessors in a computer—interpreting complex information and generating complex responses.
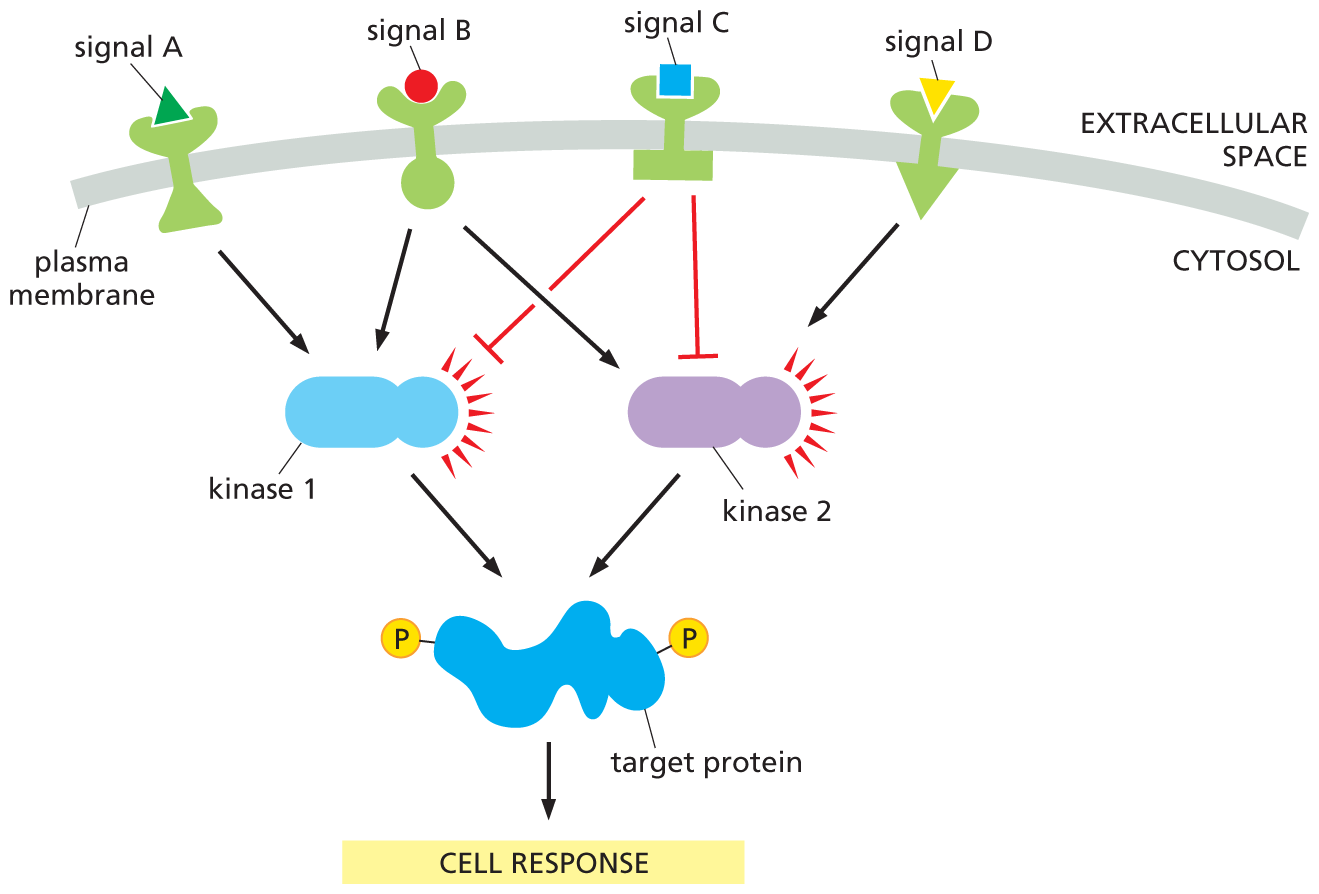
A flow diagram shows how intracellular signaling proteins integrate incoming signals. Four extracellular signal molecules, labeled as A, B, C, and D, are attached to the receptors in the plasma membrane. Signals A and B activate kinase 1. Signal C inhibits kinase 1 and kinase 2. Signal D activates kinase 2. Kinase 1 and 2 activate the target protein bound to two phosphates, triggering a cell response.
Figure 16–44 Intracellular signaling proteins serve to integrate incoming signals. Extracellular signals A, B, C, and D activate different receptors in the plasma membrane. The receptors act upon two protein kinases, which they either activate (black arrow) or inhibit (red crossbar). The kinases phosphorylate the same target protein and, when it is fully phosphorylated, this target protein triggers a cell response. It can be seen that signal molecule B activates both protein kinases and therefore produces a strong output response. Signals A and D each activate a different kinase and therefore produce a response only if they are simultaneously present. Signal molecule C inhibits the cell response and will compete with the other signal molecules. The net outcome will depend both on the numbers of signaling molecules and the strengths of their connections. In a real cell, these parameters would be determined by evolution.
Our understanding of these intricate networks is still evolving: we are still discovering new links in the chains, new signaling partners, new connections, and even new pathways. Unraveling the intracellular signaling pathways—in both animals and plants—is one of the most active areas of research in cell biology, and new discoveries are being made every day. Genome sequencing projects continue to provide long lists of components involved in signal transduction in a large variety of organisms. Yet even if we could identify every single component in this elaborate network of signaling pathways, it will remain a major challenge to figure out exactly how they all work together to allow cells—and organisms—to integrate the diverse array of information that inundates them constantly and to respond in a way that enhances their ability to adapt and survive.