QUESTION 3–1
Consider the equation
light energy + CO2 + H2O ⟶ sugars + O2 + heat energy
Would you expect this reaction to occur in a single step? Why must heat be generated in the reaction? Explain your answers.
Left to themselves, nonliving things eventually become disordered: buildings crumble and dead organisms decay. Living cells, by contrast, not only maintain but actually generate order at every level, from the large-scale structure of a butterfly or a flower down to the organization of the molecules that make up such organisms (Figure 3–3). This property of life is made possible by elaborate molecular mechanisms that extract energy from the environment and convert it into the energy stored in chemical bonds in cells. Biological structures are therefore able to maintain their form, even though the materials that form them are continually being broken down, replaced, and recycled. Your body has the same basic structure it had 10 years ago, even though you now contain atoms that, for the most part, were not part of your body then.
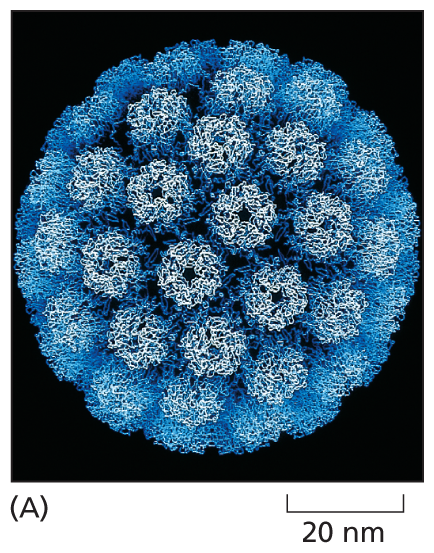
A micrograph shows the structure of a viral capsid about 50 nanometers in diameter. The structure is spherical, made of several identical protein subunits that resemble hexagons.
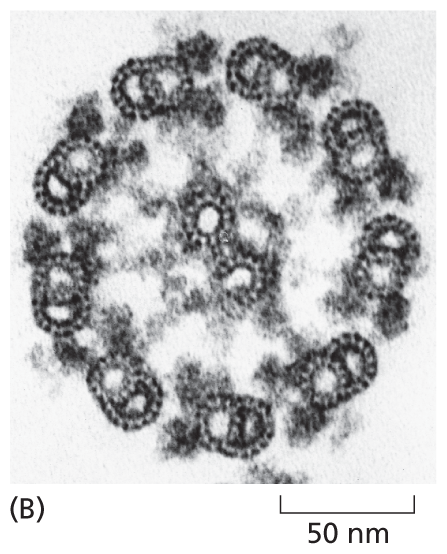
A micrograph shows the orderly arrangement of microtubules in the cross section of a sperm tail bout 100 nanometers in diameter. The section is circular.
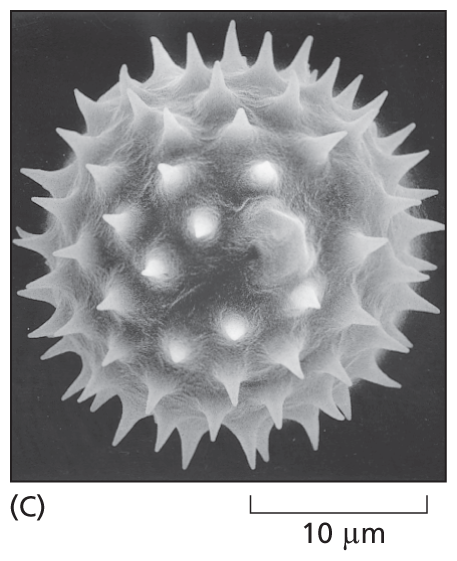
A micrograph shows the structure of a spherical pollen grain about 20 micrometers in diameter which has spikes on the outer surface.
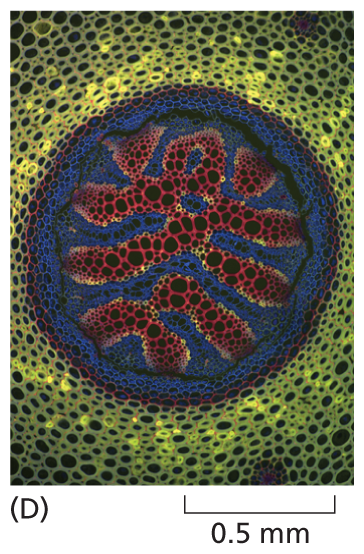
A micrograph shows the cross sectional view of a fern stem about 1 millimeter in diameter in which the cells are well defined and arranged in a circular pattern.

A micrograph shows a succulent cactus flower in which the leaves are arranged spirally. Each leaf is about 20 millimeters wide.
The universal tendency of things to become disordered is expressed in a fundamental law derived from a branch of physics called thermodynamics. According to this law, the second law of thermodynamics, disorder can only increase—in the universe as a whole or in any isolated system (a collection of matter that is completely cut off from the rest of the universe). The second law of thermodynamics has such profound implications for living things that it is worth restating in several ways.
We can express the second law in terms of probability by stating that systems will change spontaneously toward those arrangements that have the greatest probability. Consider a box in which 100 coins are all lying heads up. A series of events that disturbs the box—for example, jiggling it back and forth—will tend to move the arrangement toward a mixture of 50 heads and 50 tails. The reason is simple: there is a huge number of possible arrangements of the individual coins that can achieve the 50–50 result, but only one possible arrangement that keeps them all oriented heads up. Because the 50–50 mixture accommodates a greater number of possibilities and places fewer constraints on the orientation of each individual coin, we say that it is more “disordered.” For the same reason, one’s living space will become increasingly disordered without an intentional effort to keep it organized. Movement toward disorder is a spontaneous process, and requires a periodic input of energy to reverse it (Figure 3–4).
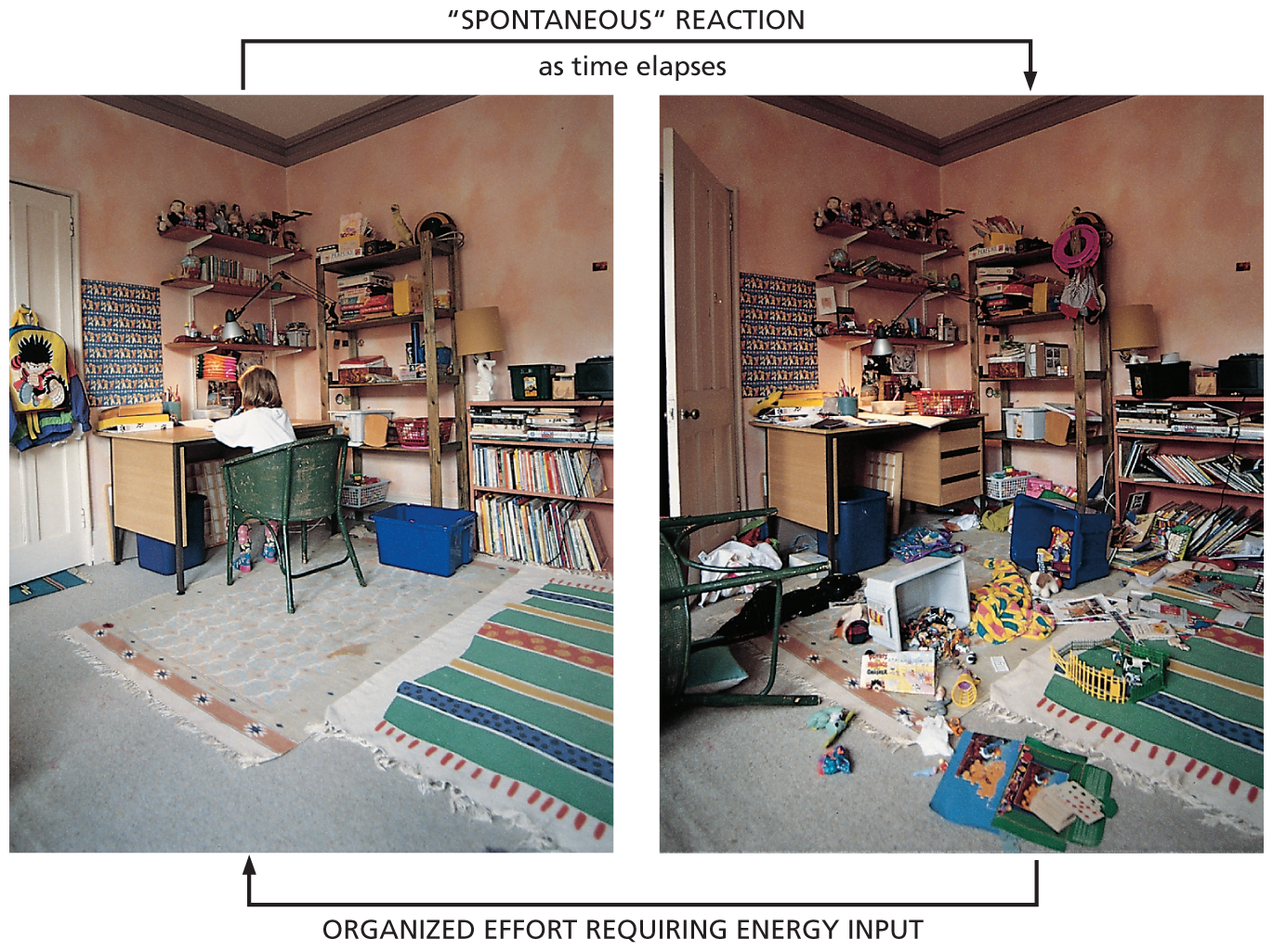
Two photos depict the effects of a spontaneous reaction and the organized effort to reverse it. The first photo shows a girl studying in a tidy room with clothes, books and possessions well-arranged and in their respective places. As time elapses, the room turns messy as depicted in the second photo, with the things scattered all around. To transform it back to a tidy room as in the first photo, an organized effort requiring energy input is needed.
Figure 3–4 The spontaneous tendency toward disorder is an everyday experience. Reversing this natural tendency toward disorder requires an intentional effort and an input of energy. In fact, from the second law of thermodynamics, we can be certain that the human intervention required will release enough heat to the environment to more than compensate for the reestablishment of order in this room.
The measure of a system’s disorder is called the entropy of the system, and the greater the disorder, the greater the entropy. Thus another way to express the second law of thermodynamics is to say that systems will change spontaneously toward arrangements with greater entropy. Living cells—by surviving, growing, and forming complex communities and even whole organisms—generate order and thus might appear to defy the second law of thermodynamics. This is not the case, however, because a cell is not an isolated system. Rather, a cell takes in energy from its environment—in the form of food, inorganic molecules, or photons of light from the sun—and uses this energy to generate order within itself, forging new chemical bonds and building large macromolecules. In the course of performing the chemical reactions that generate order, some energy is inevitably lost in the form of heat (see Figure 3–2). Heat is energy in its most disordered form: the random jostling of molecules (analogous to the random jostling of the coins in the box). Because the cell is not an isolated system, the heat energy produced by metabolic reactions is quickly dispersed into the cell’s surroundings. This dispersed energy increases the intensity of the thermal motions of molecules, thereby increasing the entropy of the cell’s environment (Figure 3–5).
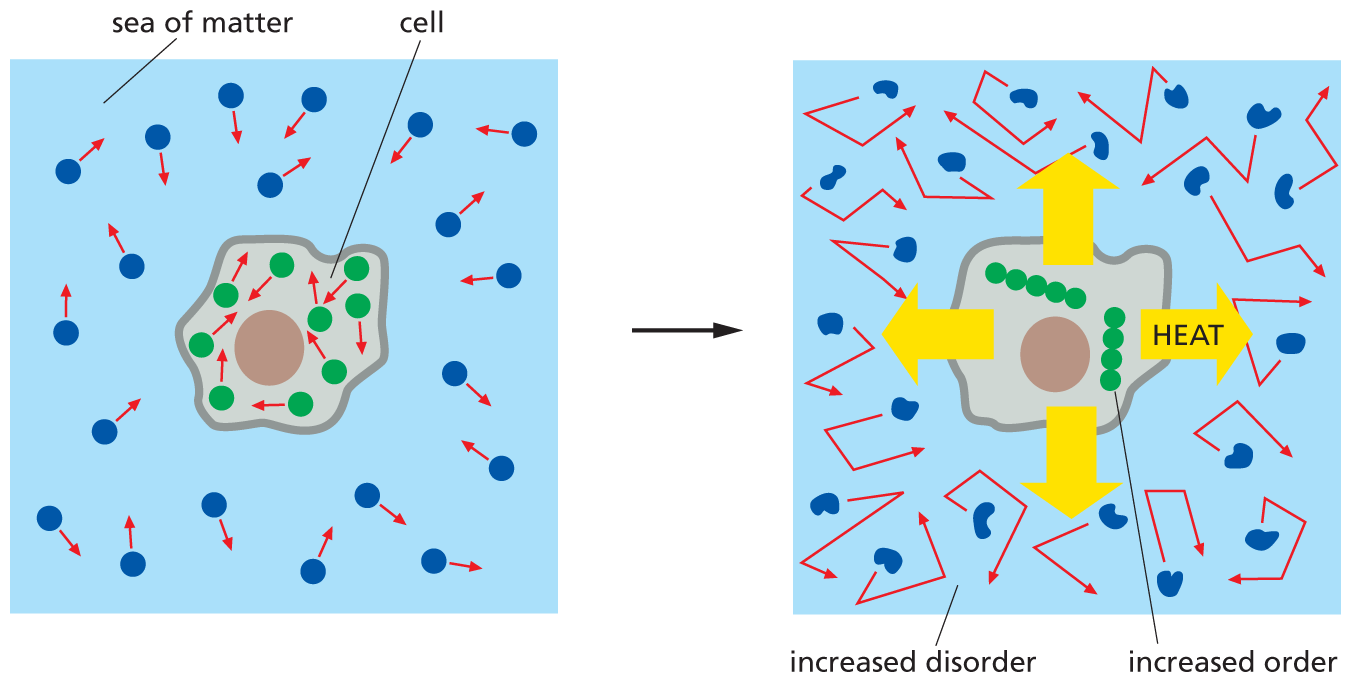
A two-part illustration depicts the second law of thermodynamics in living cells. The first illustration shows a cell surrounded by a sea of matter. The molecules inside and outside the cell are arranged in a disorderly pattern. The second illustration shows heat transferred from the cell to the sea of matter, increasing disorder outside the cell; whereas the molecules inside the cell have increased order and form two chains.
Figure 3–5 Living cells do not defy the second law of thermodynamics. In the diagram on the left, the molecules of both the cell and the rest of the universe (the environment) are depicted in a relatively disordered state. In addition, red arrows suggest the relative amount of thermal motion of the molecules both inside and outside the cell. In the diagram on the right, the cell has taken in energy from food molecules, carried out a reaction that gives order to the molecules that the cell contains (green), and released heat (yellow arrows) into the environment. The released heat increases the disorder in the cell’s surroundings—as depicted here by the increase in thermal motion of the molecules in the environment and the distortion of those molecules due to enhanced vibration and rotation. The second law of thermodynamics is thereby satisfied, even as the cell grows and constructs larger molecules.
To satisfy the second law of thermodynamics, the amount of heat released by a cell must be great enough that the increased order generated inside the cell is more than compensated for by the increased disorder generated in the environment. In other words, the chemical reactions inside a cell must increase the total entropy of the entire system: that of the cell plus its environment. Thanks to the cell’s activity, the universe thereby becomes more disordered—and the second law of thermodynamics is obeyed.
Where does the heat released by cells as they generate order come from? To understand that, we need to consider another important physical law. According to the first law of thermodynamics, energy cannot be created or destroyed—but it can be converted from one form to another (Figure 3–6). Cells take advantage of this law of thermodynamics, for example, when they convert the energy from sunlight into the energy in the chemical bonds of sugars and other small organic molecules during photosynthesis. Although the chemical reactions that power such energy conversions can change how much energy is present in one form or another, the first law tells us that the total amount of energy in the universe must always be the same.
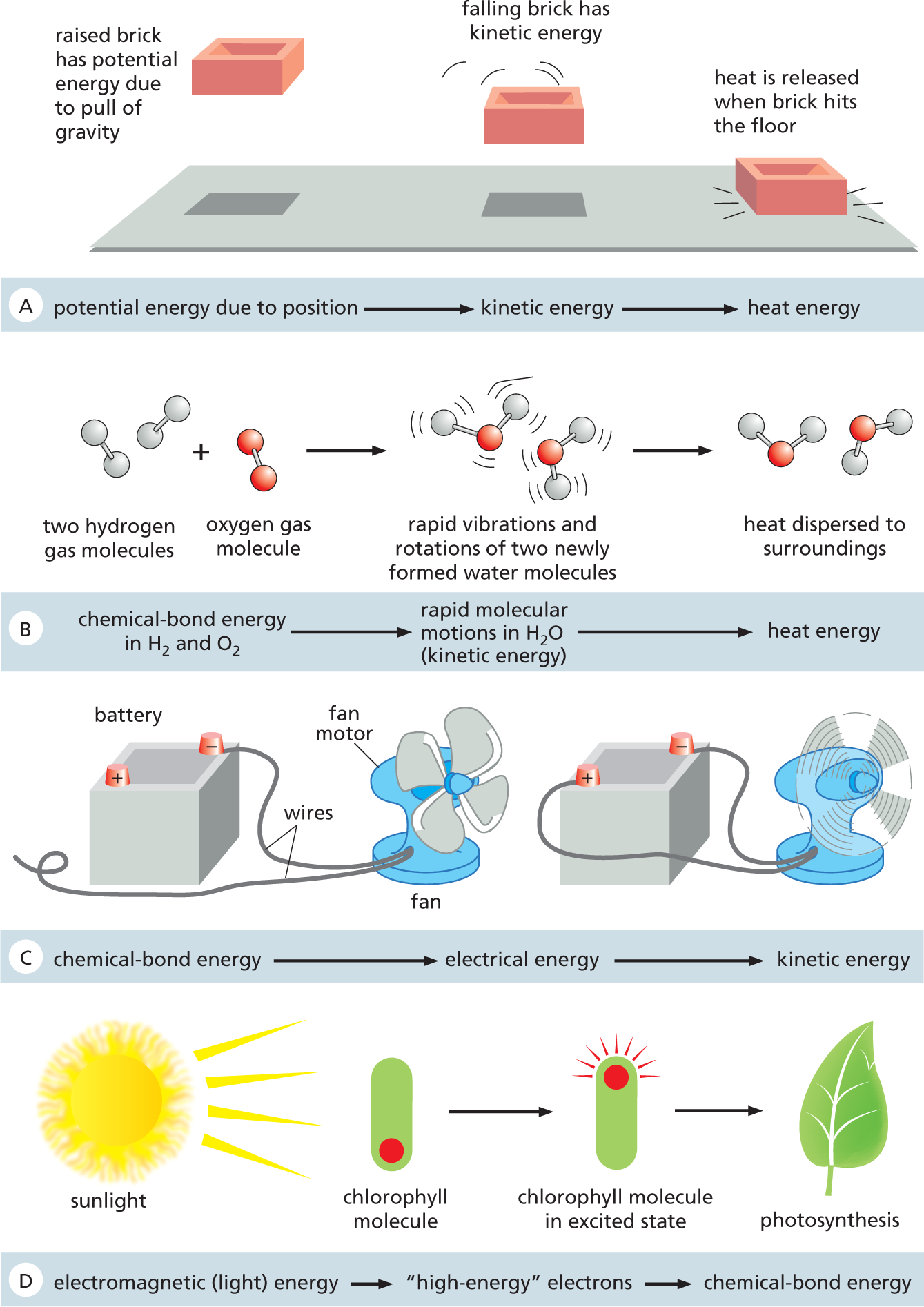
A four-part illustration depicts four different reactions. Reaction A: Potential energy due to position leads to kinetic energy which leads to heat energy. Reaction B: Chemical-bond energy in water leads to rapid molecular motions in water (kinetic energy) which leads to heat energy. Reaction C: Chemical-bond energy leads to electrical energy which leads to kinetic energy. Reaction D: Electromagnetic (light energy) leads to high-energy electrons which lead to chemical-bond energy.
Figure 3–6 Different forms of energy are interconvertible, but the total amount of energy must be conserved. (A) We can use the height and weight of the brick to predict exactly how much heat will be released when it hits the floor. (B) The large amount of chemical-bond energy released when water (H2O) is formed from H2 and O2 is initially converted to very rapid thermal motions in the two new H2O molecules; however, collisions with other H2O molecules almost instantaneously spread this kinetic energy evenly throughout the surroundings (heat transfer), making the new H2O molecules indistinguishable from all the rest. (C) Cells can convert chemical-bond energy into kinetic energy to drive, for example, molecular motor proteins; however, this occurs without the intermediate conversion of chemical energy to electrical energy that a man-made appliance such as this fan requires. (D) Some cells can also harvest the energy from sunlight to form chemical bonds via photosynthesis.
Heat, too, is a product of energy conversion. When an animal cell breaks down foodstuffs, some of the potential energy in the chemical bonds of the food molecules (chemical-bond energy) is converted into the thermal motion of molecules (heat energy). This conversion of chemical energy into heat energy causes the universe as a whole to become more disordered—as required by the second law of thermodynamics. But a cell cannot derive any benefit from the heat energy it produces unless the heat-generating reactions are directly linked to processes that maintain molecular order inside the cell. It is the tight coupling of heat production to an increase in order that distinguishes the metabolism of a cell from the wasteful burning of fuel in a fire. Later in this chapter, we illustrate how this coupling occurs. For the moment, it is sufficient to recognize that—by directly linking the “burning” of food molecules to the generation of biological order—cells are able to create and maintain an island of order in a universe tending toward chaos.
All organisms live on the potential energy stored in the chemical bonds of organic molecules; these same molecules also provide the atoms that organisms need to construct new living matter. For animals, these molecules are obtained from food—whether that food comes from eating plants or eating other animals. Plants, by contrast, obtain energy directly from sunlight. Thus, the energy animals obtain by eating plants—or by eating animals that have eaten plants—ultimately comes from the sun (Figure 3–7).

Figure 3–7 With few exceptions, the radiant energy of sunlight sustains all life. Trapped by plants and some microorganisms through photosynthesis, light from the sun is the ultimate source of all energy for humans and other animals. (Wheat Field with a Reaper by Vincent van Gogh, 1889, Van Gogh Museum, Amsterdam. Courtesy of Vincent van Gogh Foundation.)
Solar energy enters the living world through photosynthesis, a process that converts the electromagnetic energy in sunlight into chemical-bond energy in cells. Photosynthetic organisms—including plants, algae, and some bacteria—use the energy they derive from sunlight to synthesize small chemical building blocks such as sugars, amino acids, nucleotides, and fatty acids. These small molecules in turn are converted into the macromolecules—the proteins, nucleic acids, and polysaccharides—that form the plant.
We describe the elegant mechanisms that underlie photosynthesis in detail in Chapter 14. Generally speaking, the reactions of photosynthesis take place in two stages. In the first stage, energy from sunlight is captured and transiently stored as chemical-bond energy in specialized molecules called activated carriers, which we discuss in more detail later in this chapter. All of the oxygen (O2) in the air we breathe is generated by the splitting of water molecules during this first stage of photosynthesis.
QUESTION 3–1
Consider the equation
light energy + CO2 + H2O ⟶ sugars + O2 + heat energy
Would you expect this reaction to occur in a single step? Why must heat be generated in the reaction? Explain your answers.
In the second stage, the activated carriers are used to help drive a carbon-fixation process, in which sugars are manufactured from carbon dioxide gas (CO2). In this way, photosynthesis generates an essential source of stored chemical-bond energy and other organic materials—for the plant itself and for any animals that eat it. Together, the two stages of photosynthesis carry out the reaction CO2 + H2O ⟶ sugars + O2 (Figure 3–8).
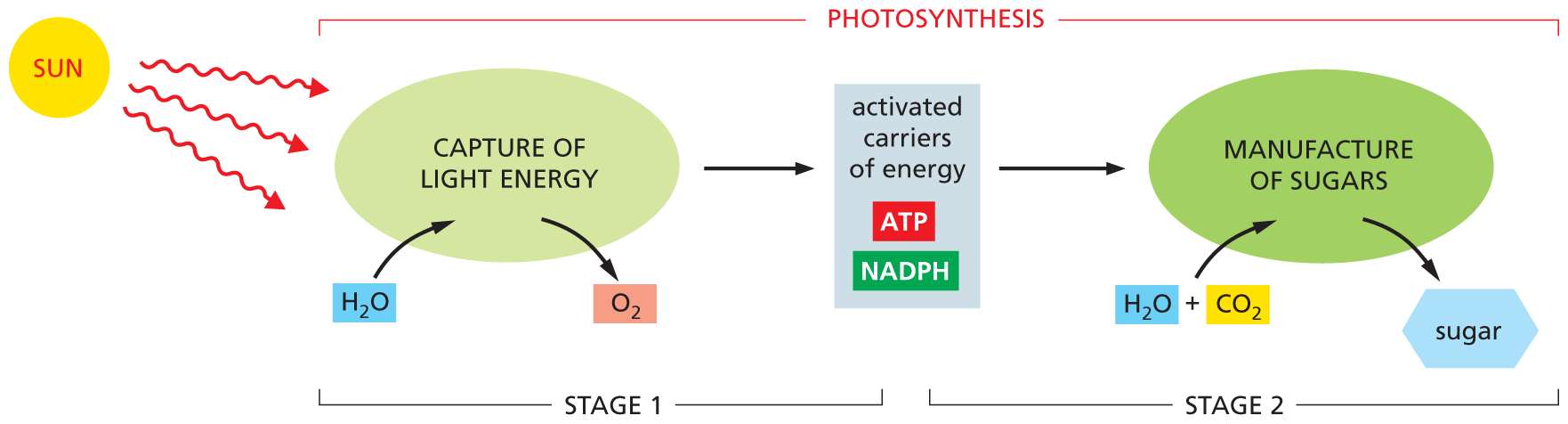
An illustration depicts stages 1 and 2 of photosynthesis. In Stage 1, the light energy for photosynthesis is captured from the sun in the presence of water molecules (H subscript 2 O); oxygen molecules are released. This activates the carriers of energy (A T P and N A D P H). In Stage 2, the activated carriers of energy lead to manufacture of sugars by the addition of water molecules (H subscript 2 O) and carbon dioxide (C O subscript 2).
Figure 3–8 Photosynthesis takes place in two stages. The activated carriers generated in the first stage, ATP and NADPH, are described in detail later in this chapter.
To live, grow, and reproduce, all organisms extract energy from the chemical bonds of organic molecules—either the sugars that a plant has produced by photosynthesis as food for itself or the mixture of large and small molecules that an animal has eaten. In both plants and animals, this extraction of chemical energy from food molecules takes place through a process of gradual oxidation.
Earth’s atmosphere is about 21% oxygen. Under these conditions, the most energetically stable form of carbon is CO2 and that of hydrogen is H2O. The oxidation of food molecules ultimately produces CO2 and H2O. So, for example: sugars + O2 ⟶ CO2 + H2O. Such reactions are therefore energetically very favorable. The complex stepwise process by which food molecules are broken down in this way to produce energy is known as cell respiration.
Cell respiration and photosynthesis are complementary processes (Figure 3–9). Plants, animals, and microorganisms have existed together on this planet for so long that they have become an essential part of each other’s environments. The oxygen released by photosynthesis is consumed by organisms—including both plants and animals—for the oxidative breakdown of organic molecules. And some of the CO2 molecules that are incorporated into sugars by photosynthesis in a green leaf were released into the atmosphere by the respiration of an animal, a fungus, or the plant itself. Carbon atoms therefore pass through a huge cycle that involves the entire biosphere—the collection of living things on Earth—as they move between organisms and the environment (Figure 3–10).
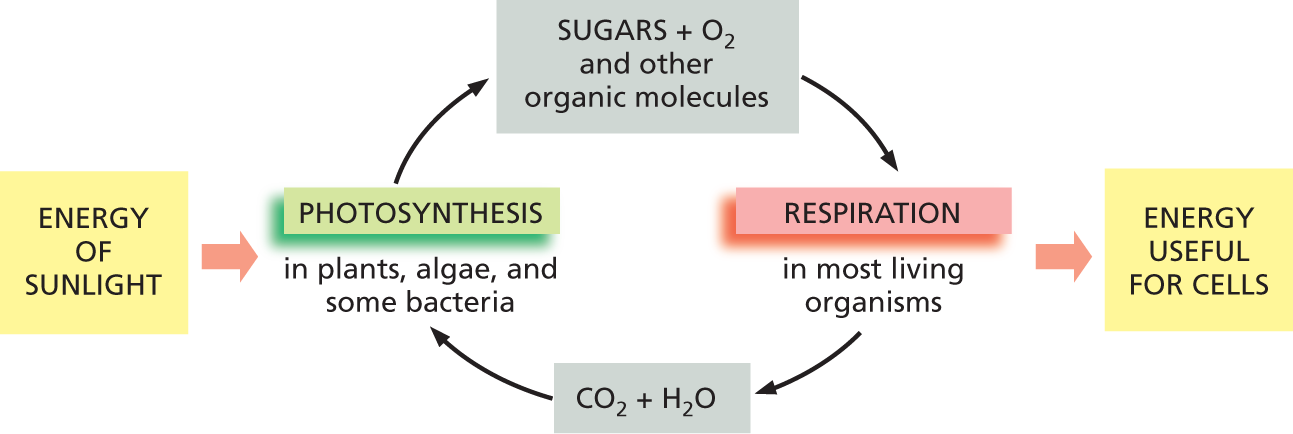
A flow chart depicts the photosynthesis and cell respiration process in living cells. The Energy of Sunlight leads to photosynthesis in plants, algae, and some bacteria. This produces sugars, oxygen, and other organic molecules which are used in respiration in most living organisms. Respiration produces energy useful for cells as well as carbon dioxide and water which are used in photosynthesis.
Figure 3–9 Photosynthesis and cell respiration are complementary processes. Photosynthesis—carried out by plants and photosynthetic organisms—uses the energy of sunlight to produce sugars and other organic molecules from the carbon atoms in CO2 in the atmosphere. Cell respiration—which takes place in most living things, including plants and other photosynthetic organisms—uses O2 to oxidize these organic molecules, releasing carbon atoms in the form of CO2 back to the atmosphere. In the process, the organisms obtain the useful chemical-bond energy that they need to survive.
Evolutionarily speaking, photosynthesis must have preceded cell respiration on the Earth, because billions of years of photosynthesis were required to release enough O2 to create an atmosphere that could support respiration, as we discuss in Chapter 14.
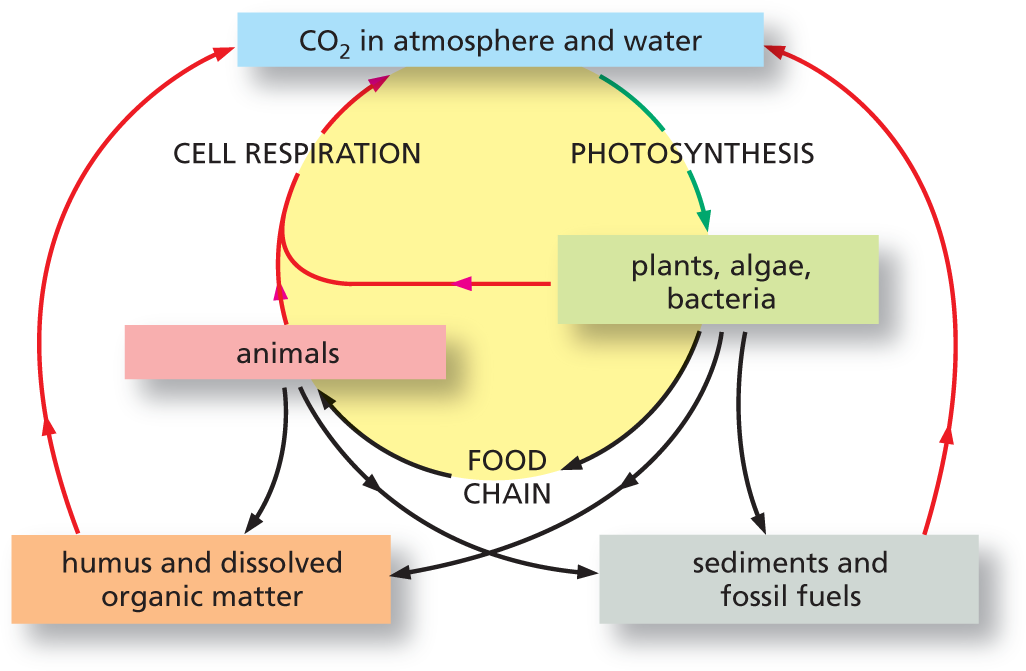
An illustration shows carbon atoms cycle. Carbon dioxide present in the atmosphere and water is used for photosynthesis on plants, algae, and bacteria. Carbon dioxide is released from cell respiration in animals as well as plants, algae, and bacteria, and joins C O subscript 2 in atmosphere and water. Plants, algae, and bacteria are also a part of the food chain and are consumed by animals. Plants, algae, and bacteria also lead to humus and dissolved organic matter which contributes to C O subscript 2 in the atmosphere and water. Plants, algae, and bacteria along with animals, form sediments and fossil fuels which contribute to C O subscript 2 in the atmosphere and water.
Figure 3–10 Carbon atoms cycle continuously through the biosphere. Individual carbon atoms are incorporated into organic molecules of the living world by the photosynthetic activity of plants, algae, and bacteria. They then pass to animals and microorganisms—as well as into organic material in soil and oceans—and are ultimately restored to the atmosphere in the form of CO2 when organic molecules are oxidized by cells during respiration or burned by humans as fossil fuels. In this diagram, the green arrow denotes an uptake of CO2, whereas the red arrows indicate CO2 release.
Both photosynthesis and cell respiration involve the use of enzyme catalysts, which direct the participating molecules through a series of chemical reactions. Both also rely on a transfer of electrons to power these chemical transformations. Reactions of this type are called oxidation–reduction reactions.
Although the term oxidation literally means the addition of oxygen atoms to a molecule, oxidation is said to occur in any reaction in which electrons are transferred between atoms. Oxidation, in this sense, involves the removal of electrons from an atom. Thus, Fe2+ is oxidized when it loses an electron to become Fe3+. The converse reaction, called reduction, involves the addition of electrons to an atom. Fe3+ is reduced when it gains an electron to become Fe2+, and a chlorine atom is reduced when it gains an electron to become Cl–.
Because the number of electrons is conserved in a chemical reaction (there is no net loss or gain), oxidation and reduction always occur simultaneously: that is, if one molecule gains an electron in a reaction (reduction), a second molecule must lose the electron (oxidation). So if an atom of iron (Fe) were to donate a pair of electrons to copper (Cu2+), the iron would become oxidized to Fe2+ and the copper would be reduced to Cu. Such electron transfers are common in photosynthesis and cell respiration, which involve enzymes carrying tightly bound metals, such as iron and copper, that facilitate the transfer of electrons.
Although it might be confusing to refer to a “gain” of electrons as “reduction,” this term arose before anything was known about the movement of electrons. The reduction reactions observed by early chemists involved a liberation of oxygen—for example, during the extraction of metals from ores. This loss of oxygen caused the samples to become lighter; in other words, “reduced” in mass.
It is important to recognize that the terms oxidation and reduction apply even when there is only a partial shift of electrons between atoms. When a carbon atom becomes covalently bonded to an atom with a strong affinity for electrons—oxygen, chlorine, or sulfur, for example—it gives up more than its equal share of electrons to form a polar covalent bond. The positive charge of the carbon nucleus now slightly exceeds the negative charge of its electrons, so that the carbon atom acquires a partial positive charge (δ+) and is said to be oxidized. Conversely, the carbon atom in a C–H bond has somewhat more than its share of electrons; it acquires a partial negative charge (δ–) and so is said to be reduced (Figure 3–11A).
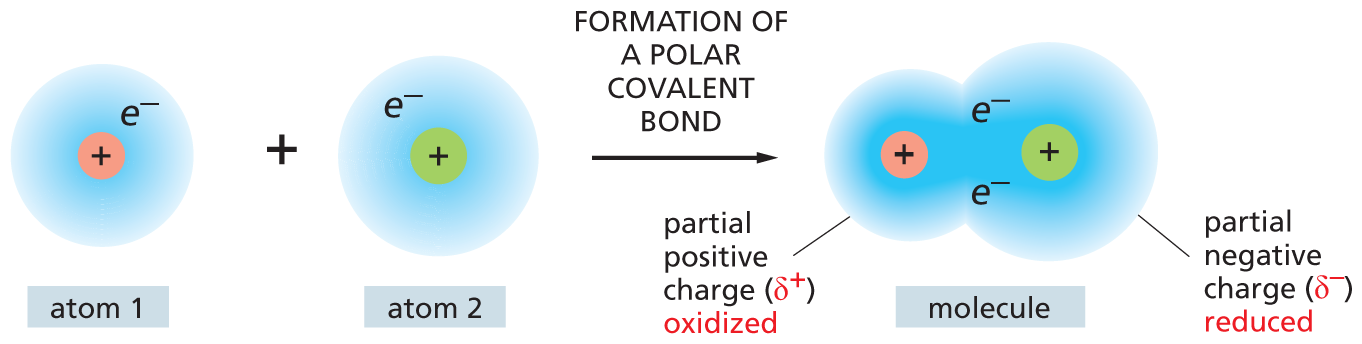
An illustration shows atom 1 (positive charge) reacting with atom 2 (positive charge) to form a polar covalent bond in the product molecule. The share of electrons for each atom is represented by a blue cloud around the atom. The atom with a lesser electron share is oxidized and the atom with a greater electron share is reduced; hence the resultant molecule has a partial positive charge and a partial negative charge.
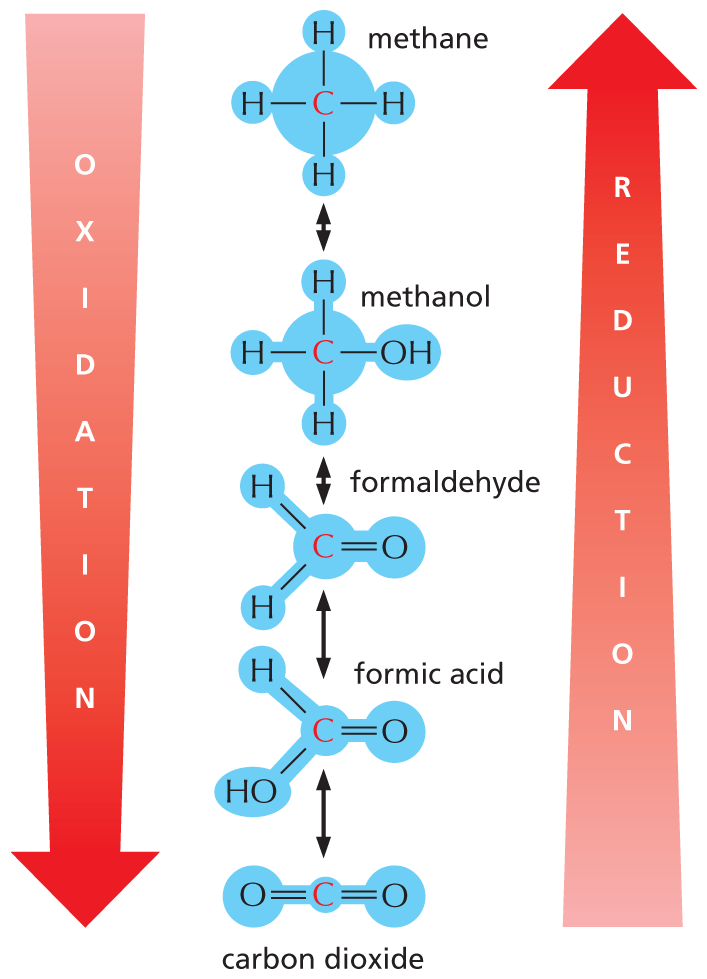
An illustration shows a stepwise oxidation of methane to form carbon dioxide and a stepwise reduction of carbon dioxide to form methane. The steps are as follows:
Oxidation: methane is oxidized to methanol in step 1; methanol is oxidized to formaldehyde in step 2; formaldehyde is oxidized to formic acid in step 3; and formic acid is oxidized to carbon dioxide in step 4.
Reduction: carbon dioxide is reduced to formic acid in step 1; formic acid is reduced to formaldehyde in step 2; formaldehyde is reduced to methanol in step 3; and methanol is reduced to methane in step 4.
The structural formulas of the compounds are as follows:
Carbon dioxide: a carbon atom double bonded to two oxygen atoms; formic acid: a central carbon atom single bonded to a hydrogen and a hydroxyl group, and double bonded to an oxygen atom.
Formaldehyde: a central carbon atom single bonded to two hydrogen atoms and double bonded to an oxygen atom; methanol: a central carbon atom single bonded to three hydrogen atoms and a hydroxyl group; methane: a central carbon atom single bonded to four hydrogen atoms.
In such oxidation–reduction reactions, electrons generally do not travel alone. When a molecule in a cell picks up an electron (e–), it often picks up a proton (H+) at the same time (protons being freely available in water). The net effect in this case is to add a hydrogen atom to the molecule:
A + e– + H+ ⟶ AH
Even though a proton is involved (in addition to the electron), such hydrogenation reactions are reductions, and the reverse dehydrogenation reactions are oxidations. An easy way to tell whether an organic molecule is being oxidized or reduced is to count its C–H bonds: an increase in the number of C–H bonds indicates a reduction, whereas a decrease indicates an oxidation (Figure 3–11B).
As we will see later in this chapter—and again in Chapter 13—cells use enzymes to catalyze the oxidation of organic molecules in small steps, through a sequence of reactions that allows much of the energy that is released to be harvested in useful forms, instead of being liberated as heat.