1.2 Why Carbon?
Why does the carbon atom play such a central role in the chemistry of life and what is so special about it? First of all, the compounds possible when carbon is their chief structural component are incredibly diverse. As we see in Section 1.6, the carbon atom can form four covalent bonds to other atoms—especially other carbon atoms.
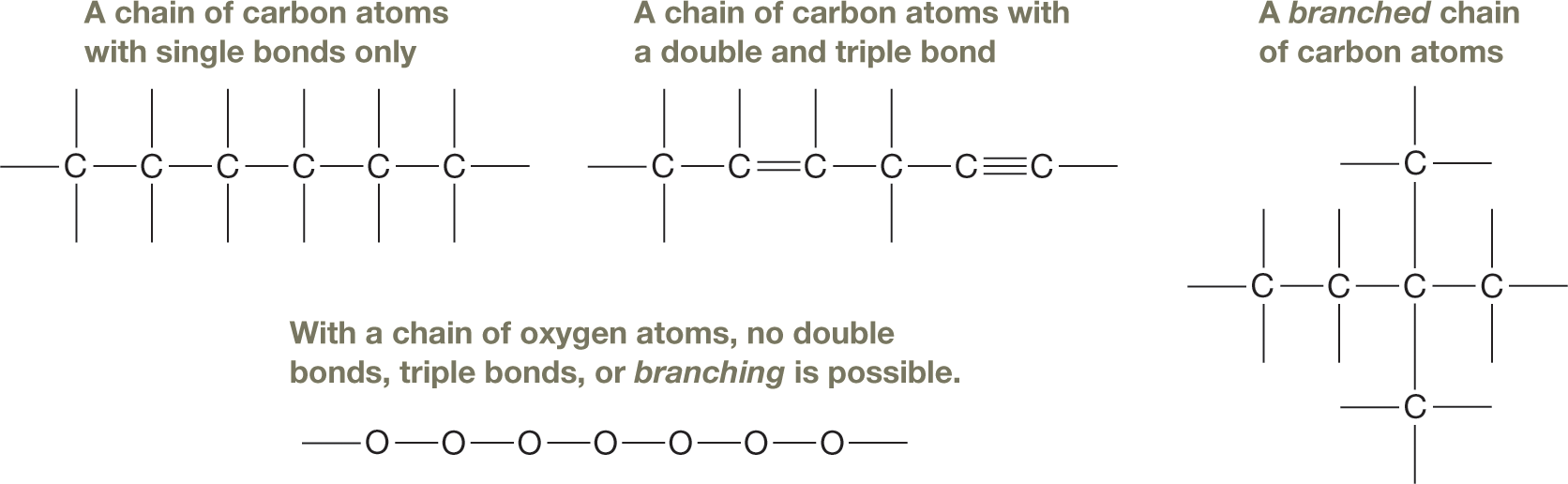
Consequently, carbon atoms can link together in chains of almost any length and rings of various sizes, allowing for an enormous range in molecular size and shape. Moreover, the ability to form four bonds means there is potential for branching at each carbon in the chain. And each carbon atom is capable of forming not only single bonds, but double and triple bonds as well. These characteristics make possible a tremendous number of compounds, even with a relatively small number of carbon atoms. Indeed, to date, tens of millions of organic compounds are known, and the list is growing rapidly as we continue to discover or synthesize new compounds.
Far less diversity would be possible in compounds based on another element, such as oxygen. Oxygen atoms tend to form two covalent bonds, which would allow for a linear chain only (as shown in the hypothetical example on p. 3). No branching could occur, nor could other groups or atoms be attached to the chain except at the ends. Furthermore, the atoms along the chain could not participate in either double or triple bonds.

If carbon works so well, then why not silicon, which appears just below carbon in the periodic table? Elements in the same group (column) of the periodic table tend to exhibit similar chemical properties, so silicon, too, can form four covalent bonds, giving it the same potential for diversity as carbon.
The answer is stability. As we see in Section 1.4, the carbon atom forms rather strong bonds with a variety of atoms, including other carbon atoms. For example, it takes 339 kJ/mol (81 kcal/mol) to break an average C—C single bond, and 418 kJ/mol (100 kcal/mol) to break an average C—H bond. By contrast, it takes only 223 kJ/mol (53 kcal/mol) to break a typical Si—Si bond. The strength of typical bonds involving carbon atoms goes a long way toward keeping biomolecules intact—an essential characteristic for molecules whose job is to store information or provide cellular structure.
Even though organic molecules are based on the carbon atom, what would life be like, hypothetically, if silicon atoms were to replace carbon atoms in biomolecules such as glucose (C6H12O6)? Glucose is broken down by our bodies through respiration to extract energy, according to the overall reaction in Equation 1-2. One of the by-products is carbon dioxide, a gas, which is exhaled from the lungs. In a world in which life is based on silicon, glucose would be Si6H12O6, and its by-product would be silicon dioxide (SiO2), as shown in Equation 1-3. Silicon dioxide, a solid, is the main component of sand; in its crystalline form, it is known as quartz (Fig. 1-3).
C6H12O6(s) + 6 O2(g) ⟶ 6 CO2(g) + 6 H2O(ℓ) (1-2)
Si6H12O6(s) + 6 O2(g) ⟶ 6 SiO2(s) + 6 H2O(ℓ) (1-3)