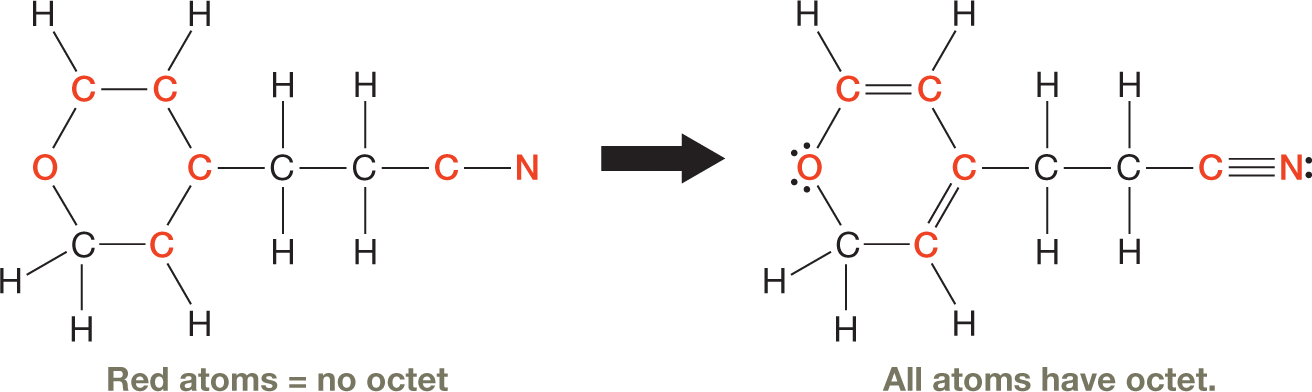
1.6 Strategies for Success: Drawing Lewis Dot Structures Quickly
After you’ve used the systematic steps (p. 13) to construct Lewis structures of several species, you may begin to notice that each type of atom tends to form a specific number of bonds and to have a specific number of lone pairs of electrons. Table 1-4 summarizes these patterns. What you will learn later is that those are the number of bonds and lone pairs for atoms that bear no formal charge. Atoms that are charged have combinations of bonds and lone pairs that are different from those in Table 1-4.
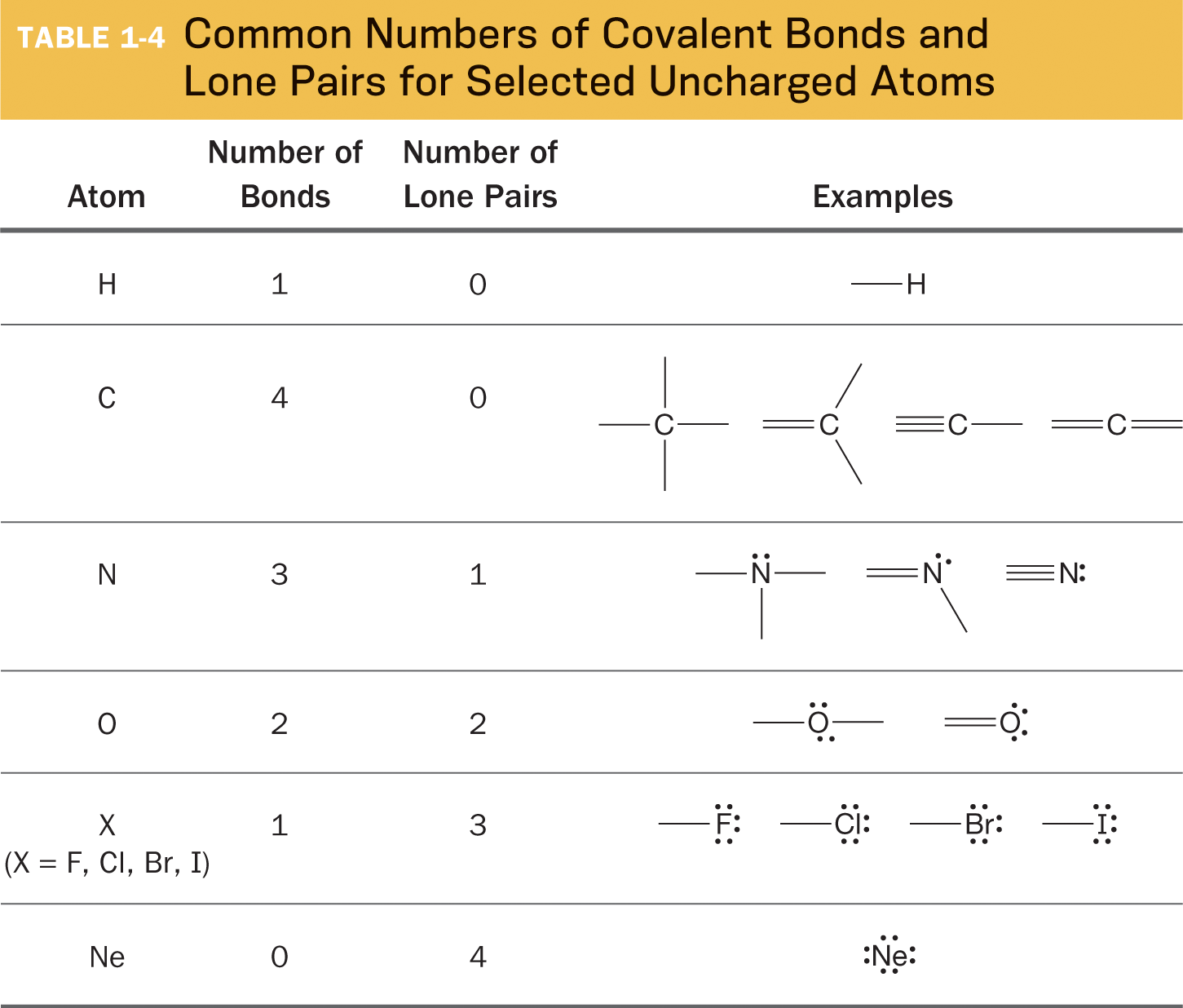
We can now use the patterns shown in Table 1-4 to complete the Lewis structure in Solved Problem 1.10.
Solved Problem 1.10
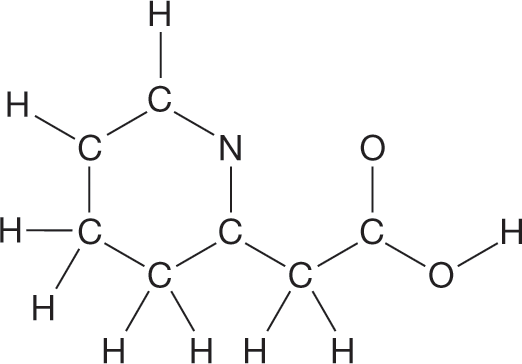
Complete the Lewis structure for the compound whose skeleton is shown at the right. Assume that all atoms are uncharged.
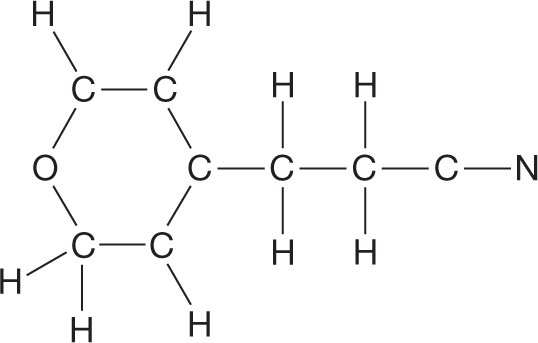
|
Think |
SHOW SECTION
|
Which atoms (other than hydrogen) have an octet and which atoms don’t? How many bonds and lone pairs are typical for each element?
|
Solve |
SHOW SECTION
|