2

2

Geckos can climb effortlessly on almost every surface. Their ability to do so is attributed to ultrafine hairs on their feet, which give rise to a very large contact surface area. This allows for rather strong dispersion forces, one of the intermolecular interactions we examine in this chapter.
On completing Chapter 2 you should be able to:
Carbon dioxide (CO2) and methanoic acid (HCO2H), also called formic acid, are similar in their chemical makeup, but the boiling point of CO2 is –78 °C, whereas that of HCO2H is 101 °C. Moreover, CO2 is only slightly soluble in water, whereas HCO2H is infinitely soluble in water. Why are their physical properties so vastly different?
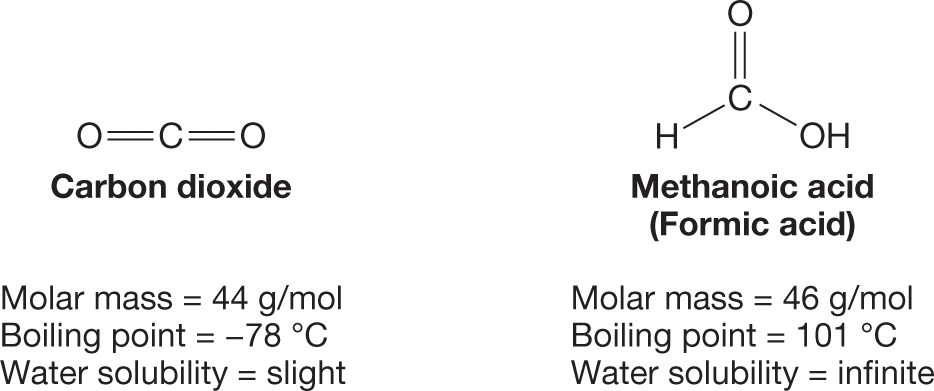
As we discuss here in Chapter 2, these compounds behave differently because they experience different intermolecular interactions. Those intermolecular interactions are governed, in turn, by a variety of factors, including the three-dimensional shapes of the molecules and the functional groups they contain. We begin, therefore, with a review of the factors that determine molecular geometry and then discuss the different types of intermolecular interactions that are important in organic chemistry.
These topics have a broad relevance to many aspects of organic chemistry. Toward the end of this chapter, we explain that intermolecular interactions determine how soaps and detergents function and contribute to the properties of cell membranes. In Chapter 5, we explain how molecular geometry is central to the important concept of chirality—that is, whether a molecule is different from its mirror image. And, in Chapter 9, we explain how intermolecular interactions can have a dramatic effect on the outcome of chemical reactions.