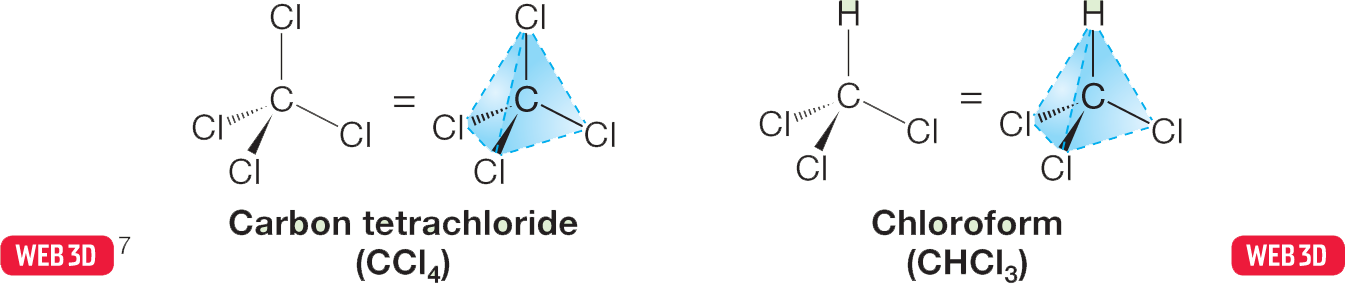
1.3 Covalent Bonds and Lewis Structures
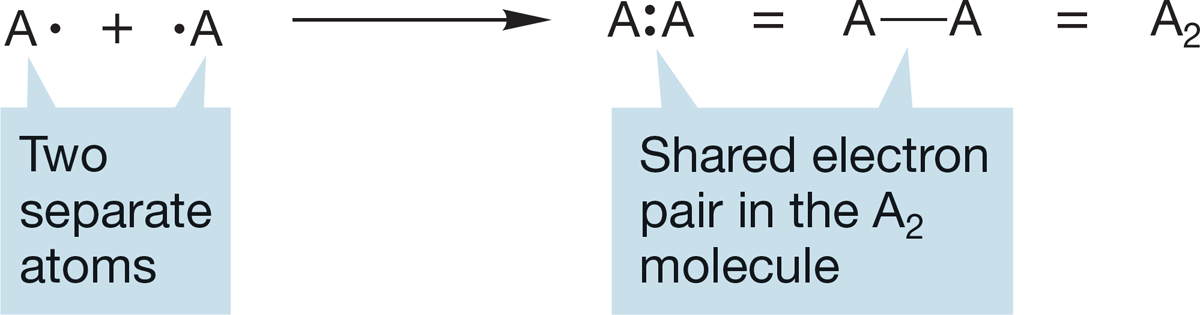
FIGURE 1.13 Bonding through the sharing of electrons is not ionic, but covalent. Covalent bonding can also result in stable electronic configurations.
The formation of molecules through covalent bonding is the subject of much of the remainder of this chapter. Because a covalent bond is formed by the sharing of a pair of electrons between two atoms, the bond is shown either as a pair of dots between two atoms or as a line joining the two (Fig. 1.13). These two-electron bonds will be prominent in almost all of the molecules shown in this book. For many simple molecules, even the line between the atoms is left out, and the compound is written simply as A2 or AB. You are left to supply mentally the missing two electrons.
The idea of covalent bonding was largely the creation of the American chemist Gilbert Newton Lewis (1875–1946), and the molecules formed by covalent bonds are usually written as what are called Lewis structures, or Lewis dot structures. In almost all covalently bonded molecules, every atom can achieve a noble gas electronic configuration by sharing electrons. In the H2 molecule, each hydrogen atom shares two electrons and thus resembles the noble gas helium (Fig. 1.14). In F2, each fluorine has a pair of electrons in the lowest-energy 1s orbital and eight other electrons in n = 2 orbitals: six unshared (called either nonbonding electrons or lone-pair electrons) and two shared in the fluorine–fluorine bond, as shown in Figure 1.14. Thus, each fluorine in F2 has a filled octet and, by virtue of sharing electrons, resembles the noble gas neon. The Lewis dot structure of HF in Figure 1.14 shows that the hydrogen is helium-like and the fluorine is neon-like.
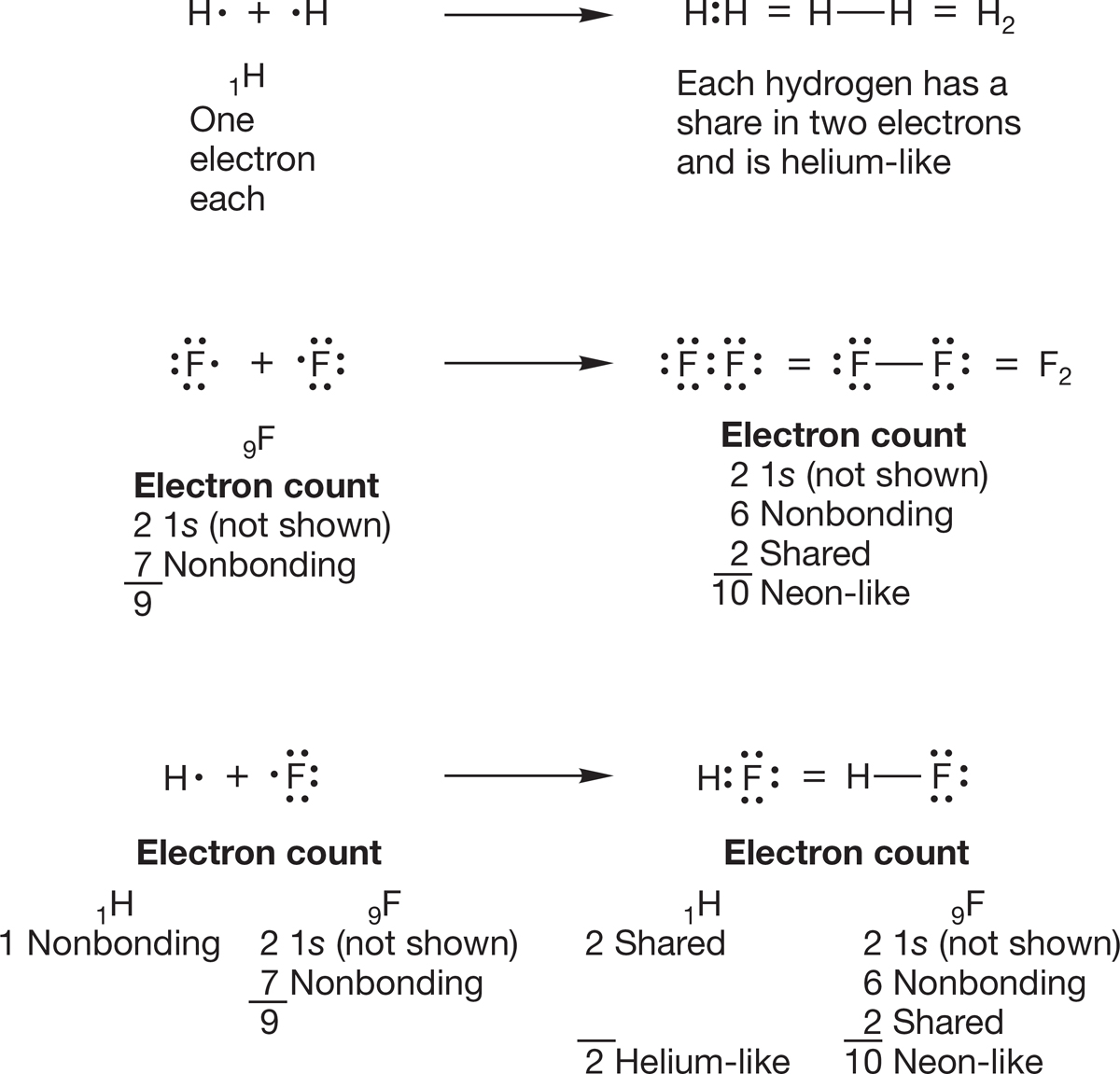
FIGURE 1.14 Lewis dot structures for H2, F2, and HF. Every atom has a noble gas electronic configuration.
With the exception of H, electrons in 1s orbitals are not involved in bonding and therefore are not shown in Lewis structures. Anytime you need to count electrons in one of these structures, you have to remember to account for the unshown 1s electrons.

FIGURE 1.15 Comparison of covalent and polar covalent bonds.
Hydrogen fluoride is different from any of the molecules mentioned so far in that the two electrons in the bond are shared unequally between the two atoms. In any diatomic molecule made from two different atoms, there cannot be equal sharing of the electrons because one atom must attract the electrons more strongly than the other atom does. Covalent bonds in which the two electrons are shared unequally are polar covalent bonds. Because electrons carry a negative charge, the atom bearing the larger share of electrons carries a partial negative charge, and the atom bearing the smaller share of electrons has a partial positive charge. Any molecule in which electrical charge is separated in this way has a dipole moment, which is a measurement of the polarity of a bond. Dipole literally means two poles. Often, a small arrow pointing from positive to negative is used to indicate the direction of the dipole or δ+ (partial positive charge) and δ− (partial negative charge) signs are placed on the appropriate atoms (Fig. 1.15). The dipole moment is measured in the unit debye (D), which was named after the Dutch physicist and chemist Petrus Josephus Wilhelmus Debye (1884–1966), who spent much of his working life at Cornell University and won the Nobel Prize in Chemistry in 1936.
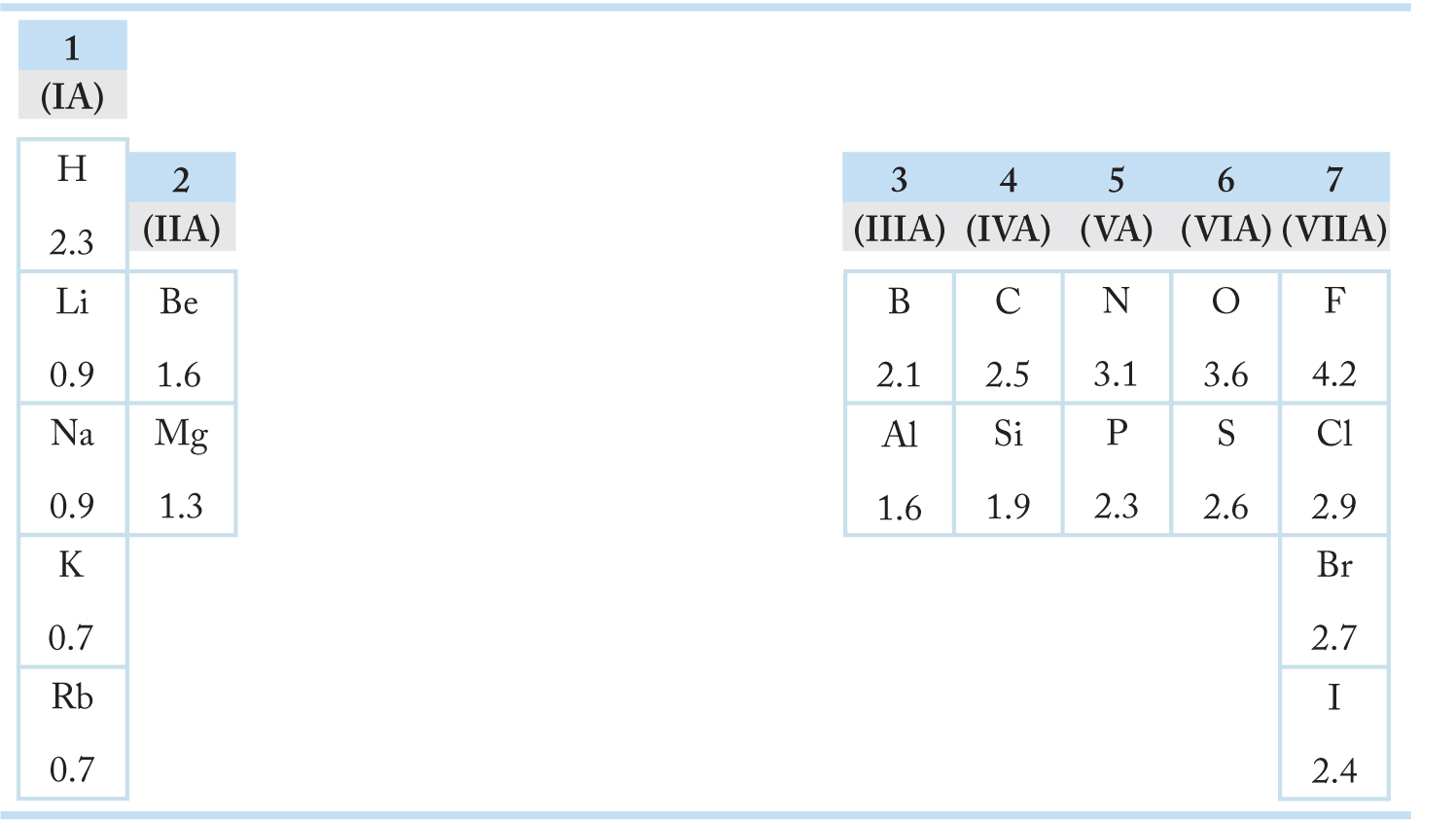
The tendency of an atom to attract electrons is called electronegativity. Atoms with high electron affinities are the most electronegative and are found at the right of the periodic table (Table 1.8). Atoms with low electronegativities and low ionization potentials are called electropositive and lie at the left of the table. The extreme case for unequal electron sharing is the ionic bonding seen in Na+F− and K+Cl−.
TABLE 1.8 Some Electronegativities
WORKED PROBLEM 1.4 Show the direction of the dipole, if there is one, in the indicated bonds of the following molecules:
H―Cl, H―F, Li―CH3, H3C―Cl, HO―NH2, H3C―CH3
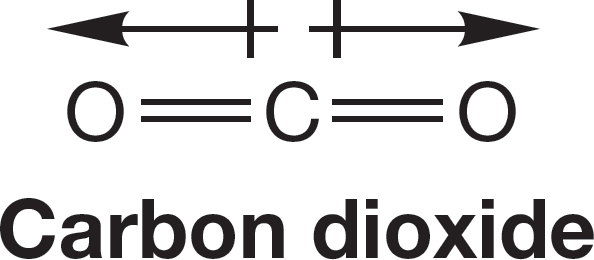
FIGURE 1.16 Carbon dioxide has no dipole moment.
Not all molecules containing polar covalent bonds have molecular dipole moments. Consider carbon dioxide (CO2), a linear molecule of the structure O C
C O (the double lines represent double bonds in which the carbon atom shares two pairs of electrons with each oxygen atom). Because oxygen is more electronegative than carbon (Table 1.8), both bonds in CO2 are polar, as shown in Figure 1.16. Even though carbon dioxide contains these two polar bonds, however, there is no molecular dipole moment because the bond dipoles cancel. The molecular dipole moment is the vector sum of all bond dipoles in the molecule. In O
O (the double lines represent double bonds in which the carbon atom shares two pairs of electrons with each oxygen atom). Because oxygen is more electronegative than carbon (Table 1.8), both bonds in CO2 are polar, as shown in Figure 1.16. Even though carbon dioxide contains these two polar bonds, however, there is no molecular dipole moment because the bond dipoles cancel. The molecular dipole moment is the vector sum of all bond dipoles in the molecule. In O C
C O the two bond dipoles are equal in magnitude and pointed in exactly opposite directions; they cancel. Carbon dioxide has two polar carbon–oxygen bonds but no molecular dipole moment.
O the two bond dipoles are equal in magnitude and pointed in exactly opposite directions; they cancel. Carbon dioxide has two polar carbon–oxygen bonds but no molecular dipole moment.
CONVENTION ALERT
Wedges and Dashes for 3D Structures
In representing three-dimensional structures on a two-dimensional surface such as a chalkboard or book page, it is necessary to devise some scheme for showing bonds directed away from the surface, either toward the front (up and out of the board or page) or toward the rear (down and into the board or page). In the figure for Problem 1.5, and in many, many future figures, the solid wedges ( ) represent covalent bonds that are coming out toward you, and the dashed wedges (
) represent covalent bonds that are coming out toward you, and the dashed wedges ( ) represent covalent bonds that are retreating into the page.
) represent covalent bonds that are retreating into the page.
PROBLEM 1.5 Which of these two molecules has a dipole moment and which does not? Look carefully at the shapes of the two tetrahedral molecules. The blue dashed lines show the outlines of a tetrahedron. If visualizing the three-dimensional aspects of these molecules is hard for you at first, by all means use molecular models.
To write Lewis dot structures for molecules containing only hydrogen and second-row atoms (most organic molecules), we need to know the number of electrons in each atom. For atoms in the second row of the periodic table, we can tell immediately how many electrons are available for bonding by knowing the atomic number of the atom or by knowing the column the atom is in. The number of available electrons is equal to the total number of electrons in the atom, which is the same as the atomic number, less the two 1s electrons. We can also use the column number, which corresponds to the number of electrons in the outer shell (see Table 1.8). The electrons in the outermost shell, which are the least tightly held electrons, are called the valence electrons.
For atoms in the third row of the periodic table, neither the two 1s electrons nor the eight electrons in the second shell (2s22p6) are shown in the Lewis structure. Instead, only the valence electrons in the third shell appear.
In complete Lewis structures, every valence electron is written as a dot. In another form of a Lewis structure, pairs of electrons forming covalent bonds are shown as lines, and only the nonbonding valence electrons appear as dots. We will use this kind of Lewis structure throughout this text. Some examples of Lewis structures are shown in Figure 1.17. Another variation of the Lewis structure uses a line to represent the nonbonding electrons; we will not use this representation because the line can easily be confused with a negative charge.
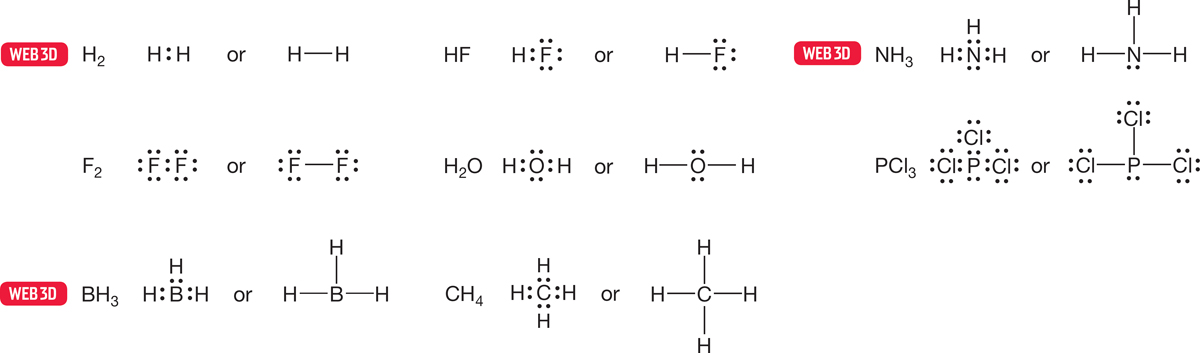
FIGURE 1.17 A few Lewis dot structures. These schematic Lewis structures do not indicate the real shapes of the molecules shown.
Let’s draw Lewis structures for methane and ammonia (Fig. 1.18). To construct the Lewis dot structure of methane (CH4), we first determine that carbon (6C) has four valence electrons for bonding. Note that carbon is in column 4 of the periodic table. Carbon has a total of six electrons, but two are in the unused 1s orbital. Each hydrogen contributes one electron. Thus, four two-electron covalent bonds are formed, with no electrons left over (Fig. 1.18a). For ammonia (:NH3), nitrogen (7N) contributes five valence electrons (seven minus the 1s pair; nitrogen is in column 5), and the three hydrogens contribute one electron each. Therefore, three two-electron nitrogen–hydrogen bonds are formed, each containing one electron from N and one from H, and there is a nonbonding pair left over (Fig. 1.18b).
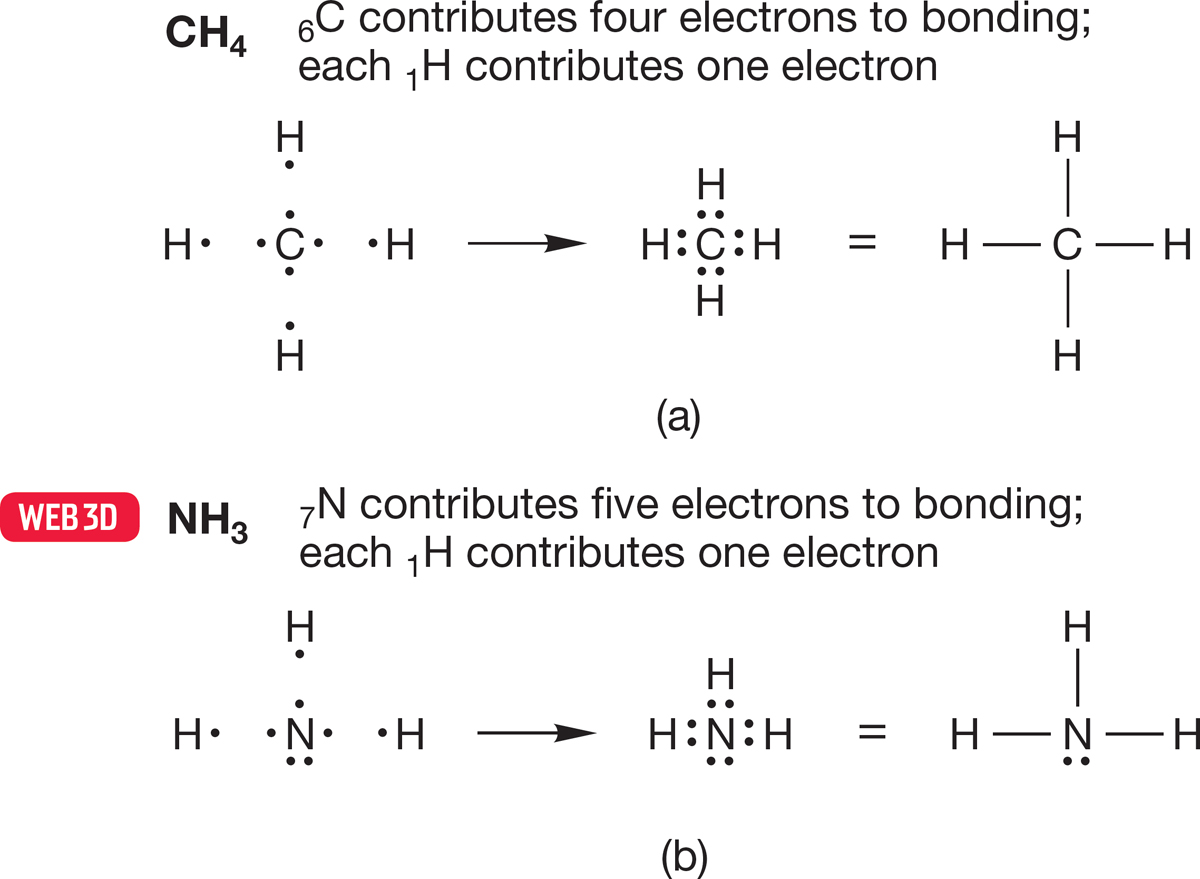
FIGURE 1.18 Construction of a Lewis structure for (a) methane, CH4, and (b) ammonia, NH3.
WORKED PROBLEM 1.6 Construct Lewis structures for the following neutral molecules:
*(a) BF3
(b) H2Be
(c) SiH4
(d) CH2Cl2
(e) HOCH3
(f) H2N―NH2
ANSWER (a) The first task is to determine the gross structure of BF3. Is it B―F―F―F, or some other structure containing fluorine–fluorine bonds, or a structure containing only boron–fluorine bonds? We start by working out the number of electrons available for bonding. Fluorine (9F) has seven valence electrons available for bonding (9 − 2 1s, or fluorine is in column 7) and thus has a single unpaired, or “odd,” electron. Boron (5B) has three valence electrons (5 − 2 1s, or boron is in column 3).

Notice that boron has three electrons available for bonding, and a structure with three boron–fluorine bonds can be nicely accommodated. There is no easy way to form a molecule containing both boron–fluorine and fluorine–fluorine bonds. A structure with covalent bonds in which the seventh electron on each fluorine is shared with one of boron’s available three electrons gives the correct answer:
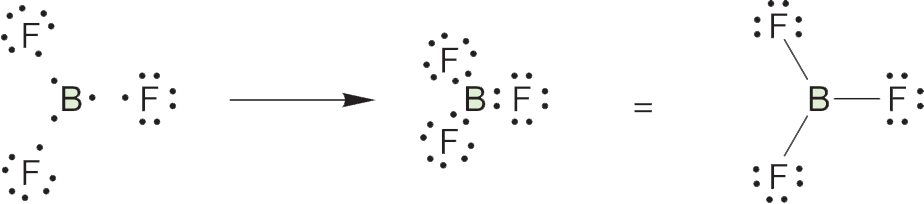
Single bonds such as the ones shown in Figures 1.17 and 1.18 are not the only kind of covalent bonds. Atoms of elements below the first row of the periodic table can form double and triple bonds, as we saw earlier when we looked at the covalent bonds in carbon dioxide. Usually, the structural formula will give you a clue when multiple bonding is necessary. For example, ethane (H3CCH3) requires no multiple bonds; indeed it permits none, as can be seen from construction of the Lewis structure (Fig. 1.19). Each carbon is attached to four other atoms—all four of the valence electrons in each carbon are shared in single bonds to the three hydrogen atoms and the other carbon. Therefore, all electrons can be accounted for in single bonds between the atoms.
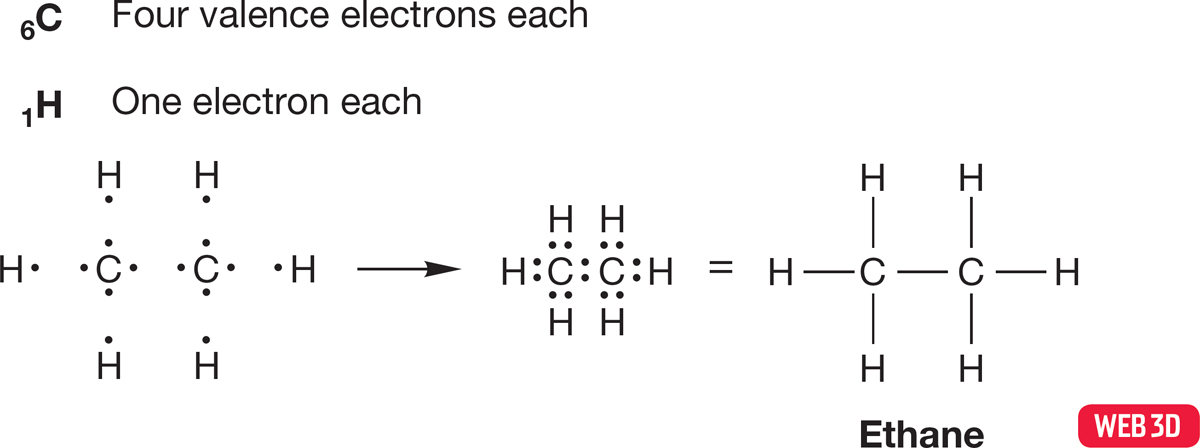
FIGURE 1.19 Construction of a Lewis structure for ethane.
In the molecule known as ethene or ethylene (H2CCH2), only three of carbon’s four bonding electrons are used up in the formation of single covalent bonds to two hydrogens and the other carbon (Fig. 1.20). An unbonded electron remains on each carbon, and these two electrons are shared in a second carbon–carbon bond. Thus, ethylene contains a carbon–carbon double bond.
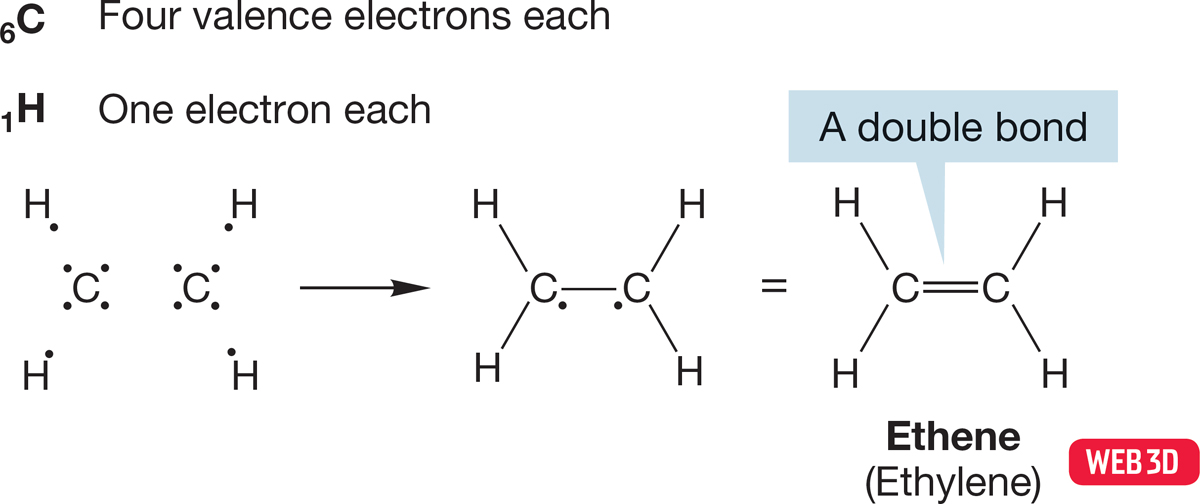
FIGURE 1.20 Construction of a Lewis structure for ethene (ethylene).
In ethyne or acetylene (HCCH), shown in Figure 1.21, two of the four bonding electrons in each carbon are used in the formation of single bonds to one hydrogen and the other carbon. Two electrons are left over on each carbon, and these are shared to create a triple bond between the carbons.
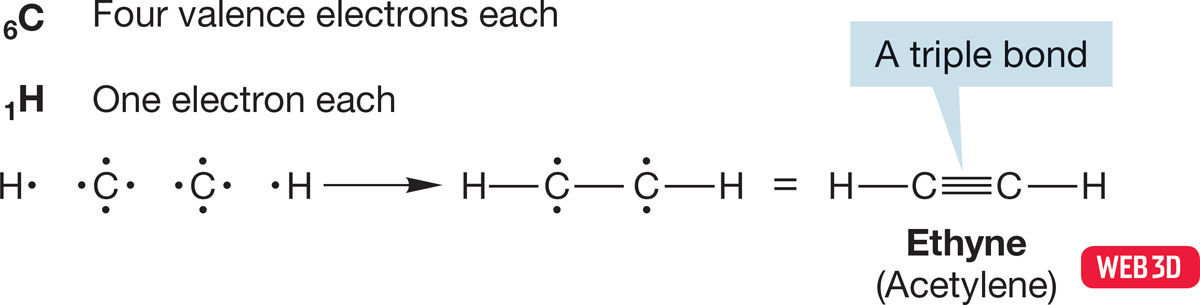
FIGURE 1.21 Construction of a Lewis structure for ethyne (acetylene).
WORKED PROBLEM 1.7 Draw Lewis structures for the following neutral species. Use lines to indicate electrons in bonds and dots to indicate nonbonding electrons.
*(a) CH3
*(b) CH2
(c) Br
(d) OH
(e) NH2
(f) H3C―N
ANSWER The first step is to determine the number of bonding electrons in each atom. Then, make the possible bonds and see how many electrons are left over. Because each compound is neutral, the number of bonding electrons is equal to the atomic number less the 1s electrons. For neutral hydrogen, there always is only the single 1s electron.
(a) Carbon has four bonding electrons and forms covalent bonds with three hydrogens, each of which contributes a single electron. There is one electron left over on carbon, which is shown as a dot in the Lewis structure:
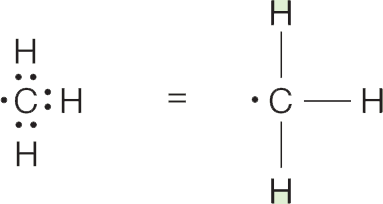
(b) In CH2, carbon can form only two covalent bonds because only two hydrogen atoms are available. Two nonbonding electrons are left over:

WORKED PROBLEM 1.8 Each of the following compounds has at least one multiple bond. Draw a Lewis structure for each molecule. Use lines to indicate electrons in bonds and dots to indicate nonbonding electrons.
(a) F2CCF2
*(b) H3CCN
(c) H2CO
(d) H2CCO
(e) H2CCHCHCH2
(f) H3CNO
(g) 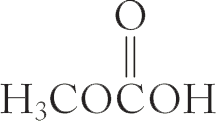
ANSWER (b) If you did Problems 1.6 and 1.7, the group known as methyl (CH3) should be familiar by now. Its three covalent bonds are constructed by sharing three of carbon’s four valence electrons with the single electrons of the three hydrogens:
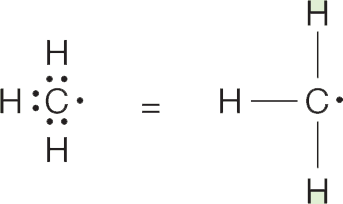
The second carbon and the nitrogen bring four and five electrons, respectively. Carbon–carbon and carbon–nitrogen covalent bonds can be formed, which leaves two unshared electrons on carbon and four on nitrogen:
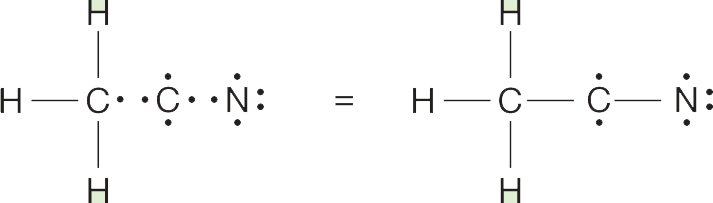
Formation of a triple bond between carbon and nitrogen completes the picture, and nitrogen is left with a nonbonding pair of electrons:
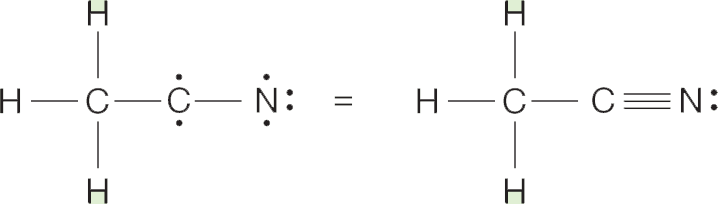
7This icon means that you can find the calculated three-dimensional structure of the molecule on the Web at wwnpag.es/orgo3dweb. That structure can be manipulated so that you can view it from any direction and changed so that you can see different versions of it (space filling, ball-and-stick, and so on). The structures can also be interrogated; they will tell you bond lengths and interatomic angles, if asked. You will also find comments, problems, and answers related to the molecule on the Web site.