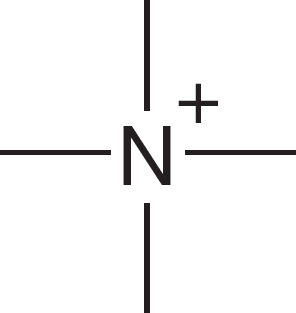
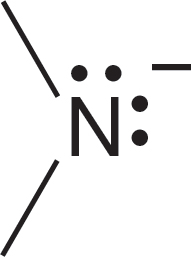
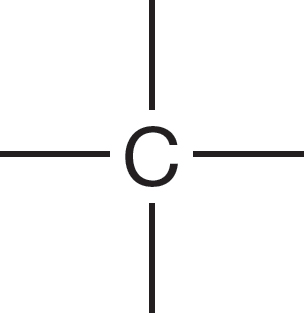
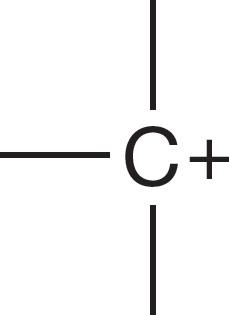
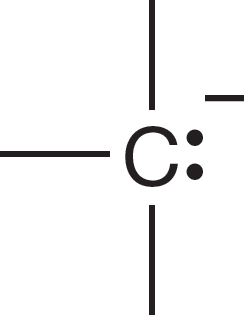
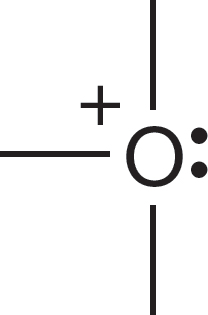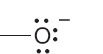1.4 Formal Charges
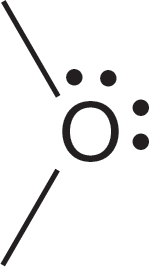
As we examine the nature of the bonding in the various molecules you will study in organic chemistry, it is vital that you be able to draw Lewis structures quickly and easily. It is also extremely important to be able to determine the charge on atoms, sometimes referred to as a formal charge, in a given structure. For single atoms in the second row of the periodic table, this process is easy as long as you remember to count the two 1s electrons, which are never shown. Figure 1.22 gives Lewis structures and charge determinations for six species. Be sure you understand how the charge is determined in each case.
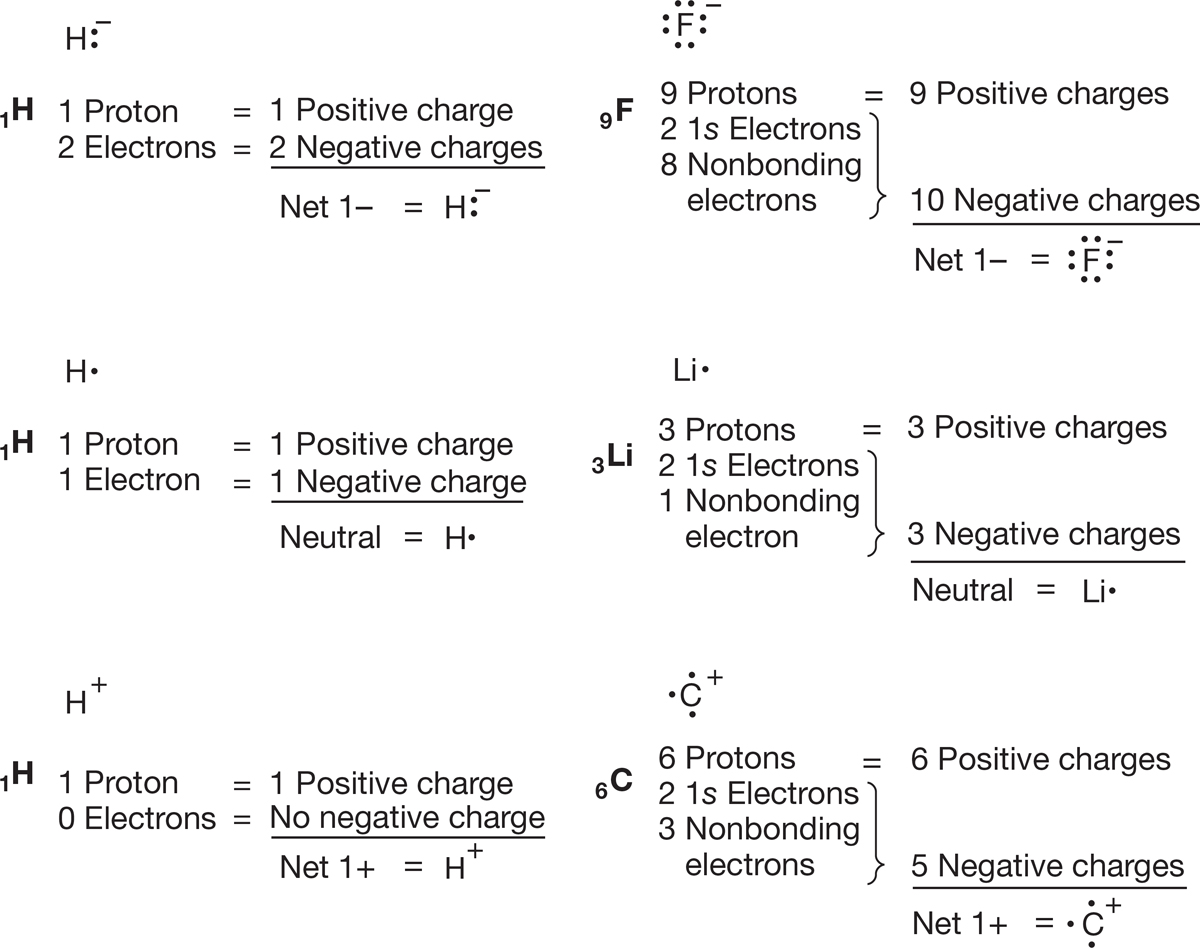
FIGURE 1.22 A few examples of charge determination.
In molecules, the determination of charge is a bit harder because you must take care to account for shared electrons in the right way. There are two steps to this process.
1. Count the electrons that the atom “owns.” Remember that every second-row atom has a pair of unshown 1s electrons. Count all nonbonding valence electrons as owned by the atom and count one electron for each bond to the atom.
2. Consider the atomic number (positive charge of the nucleus) for that particular atom and subtract the number of electrons that the atom owns (negative charges). The resulting value is the formal charge on the atom.
In H2, for example, each hydrogen has only a share in the pair of electrons binding the two nuclei, and therefore each hydrogen is neutral (Fig. 1.23). In F2, each fluorine has two 1s electrons, six nonbonding electrons, and a share in the single covalent bond between the two fluorine atoms. This count gives a total of nine electrons, exactly balancing the nine positive nuclear charges (9 − 9 = 0). In H2C H2C each carbon has a pair of 1s electrons and a share in four covalent bonds for a total of six. Because each carbon has a nuclear charge of +6, both are neutral.
H2C each carbon has a pair of 1s electrons and a share in four covalent bonds for a total of six. Because each carbon has a nuclear charge of +6, both are neutral.
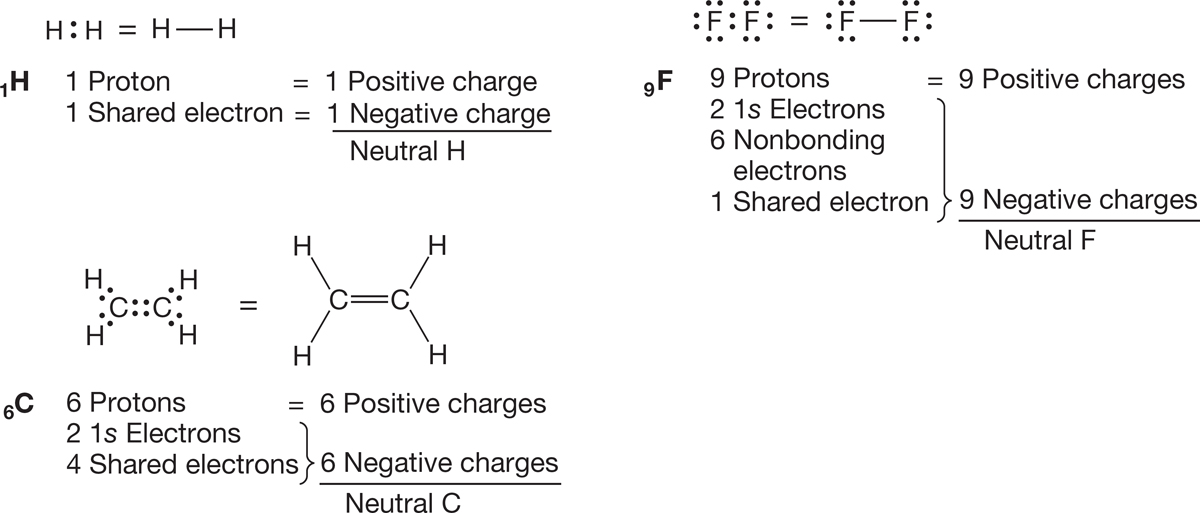
FIGURE 1.23 Electron counting in some simple molecules.
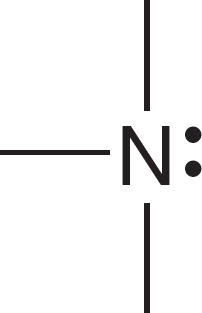
Let’s see why the carbon of the methyl anion (−:CH3) is negatively charged and why the nitrogen of the ammonium ion (+NH4) is positively charged (Fig. 1.24). In the methyl anion, carbon has a pair of 1s electrons, two nonbonding electrons, and a share in three covalent bonds, for a total of seven electrons. The nuclear charge is only +6, and therefore the carbon must be negatively charged (6 − 7 = −1). The
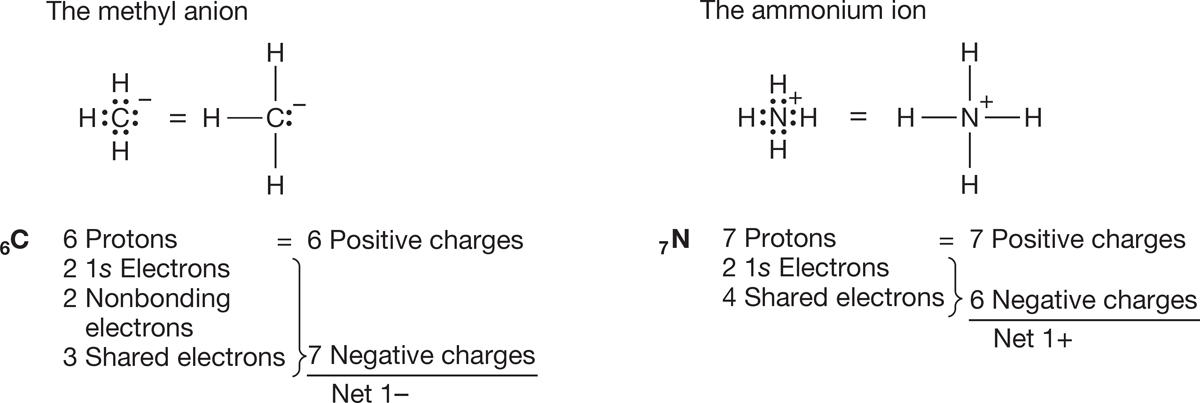
FIGURE 1.24 Examples of the calculation of the formal charge on an atom in a molecule.
nitrogen atom of the ammonium ion has two 1s electrons plus one electron from each of the four covalent bonds, for a total of six. The nuclear charge is +7, and therefore the nitrogen is positively charged (7 − 6 = +1). Figure 1.25 shows two more ions and works through the calculations of charge.

FIGURE 1.25 More calculations of formal charge in molecules.
PROBLEM 1.9 Draw Lewis structures for the following charged species. In each case, the charge is shown closest to the charged atom.
(a) −OH
(b) −BH4
(c) +NH4
(d) −Cl
(e) +CH3
(f) +OH3
(g) +NO2 (Hint: Nitrogen is the central atom.)
PROBLEM 1.10 Add charges to the following compounds wherever necessary:
(a) :CH2
(b) ·CH3
(c) 
(d) 
(e) :OH3
(f) 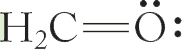
(g) 
PROBLEM 1.11 Add electrons to complete the following Lewis structures. In each case, the charge is placed as close as possible to the charged atom.
(a) +CH2
(b) −CH2CH3
(c) 
(d) +OH3
(e) −OH
(f) +NH2
(g) −NH2
(h) 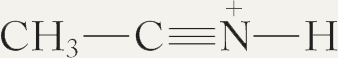
Although it is important that you be able to determine the formal charge on an atom, Table 1.9 lists frequently encountered structures containing the most important atoms in organic chemistry: H, O, N, and C.
TABLE 1.9 Lewis Structures for Some Frequently Encountered Structural Types
|
Neutral |
Positive |
Negative |
H |
|
|
|
O |
|
|
|
N |
|
|
|
C |
|
|
|
Summary
Molecules are often represented using Lewis structures. To draw a Lewis structure:
1. Draw the central atom with its valence electrons shown as dots.
2. Atom A will use one valence electron for each bond it makes to atom B. Atom A will use two of its own valence electrons if it makes a double bond to atom B or three valence electrons to make a triple bond. Hydrogen in a molecule will make one bond, oxygen typically makes two, nitrogen three, and carbon makes four bonds. Do not draw five bonds to carbon!
3. Any valence electrons that are unused in bonding are nonbonding electrons, shown as pairs of dots.
4. Determine the formal charge for each atom. A quick way to determine the formal charge on atom A is to determine the number of electrons atom A owns (one electron for each bond plus the nonbonding electrons) and subtract that from the number of valence electrons atom A should have. Any resulting formal charge should be placed next to atom A.