2.11 Drawing Isomers
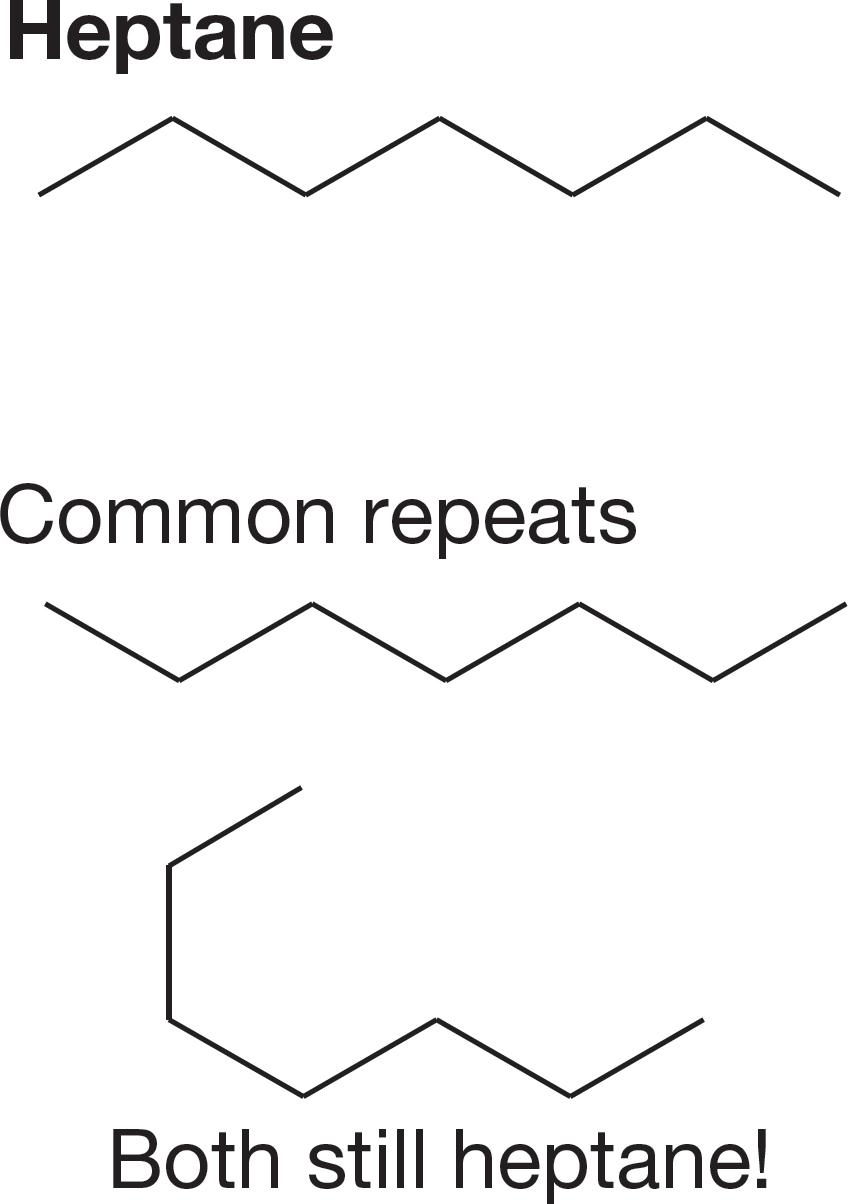
FIGURE 2.45 Heptane.
The following is a common question on organic chemistry tests: Write all the isomers of pentane (or hexane, or heptane, etc.). Such problems force us to cope with the translation of two-dimensional representations into three-dimensional reality. Figuring out the pentanes is easy; there are only three isomers. Even writing the hexane isomers (there are five) or isomers of heptane (there are nine) is not really difficult. But getting all 18 isomers of octane is a tougher proposition, and getting all 35 isomers of nonane with no repeats is a real challenge.
Success depends on finding a systematic way to write isomers. Thrashing around writing structures without a system is doomed to failure. You will not get all the isomers, and there is a high probability of generating repeat structures. Any system will work as long as it is truly systematic. One possibility is shown in the following Problem Solving box, which generates the nine isomers of heptane shown in Figures 2.45–2.48.
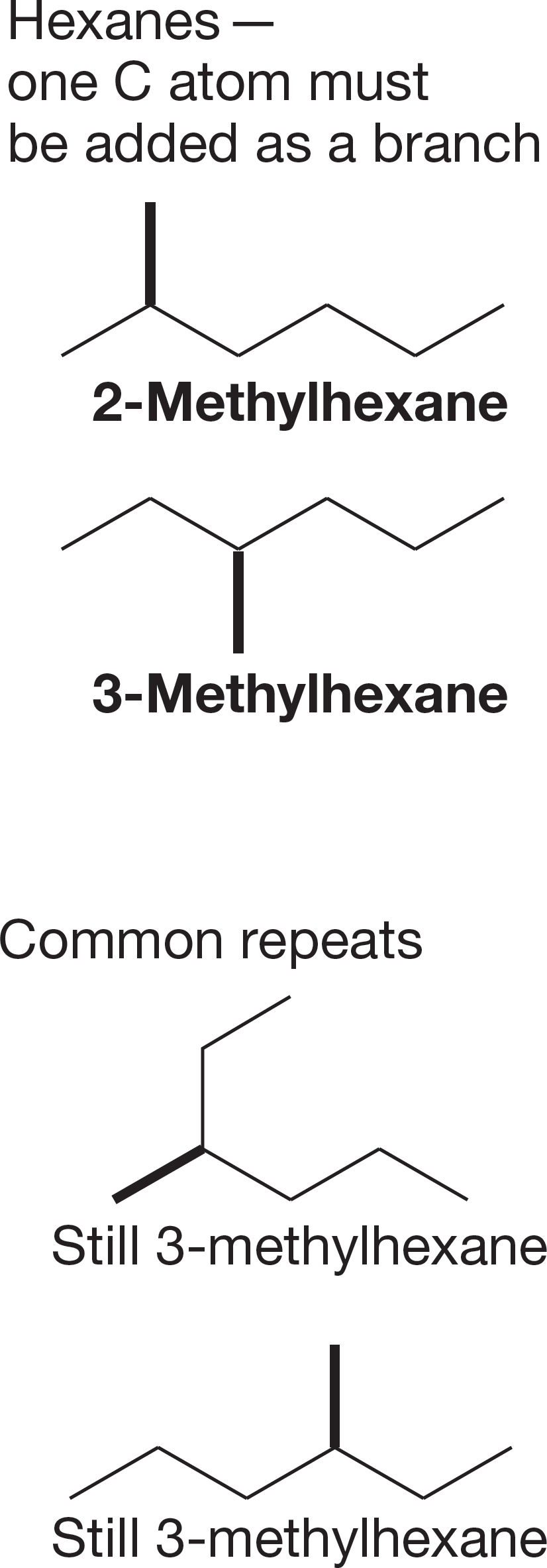
FIGURE 2.46 Methylhexanes.
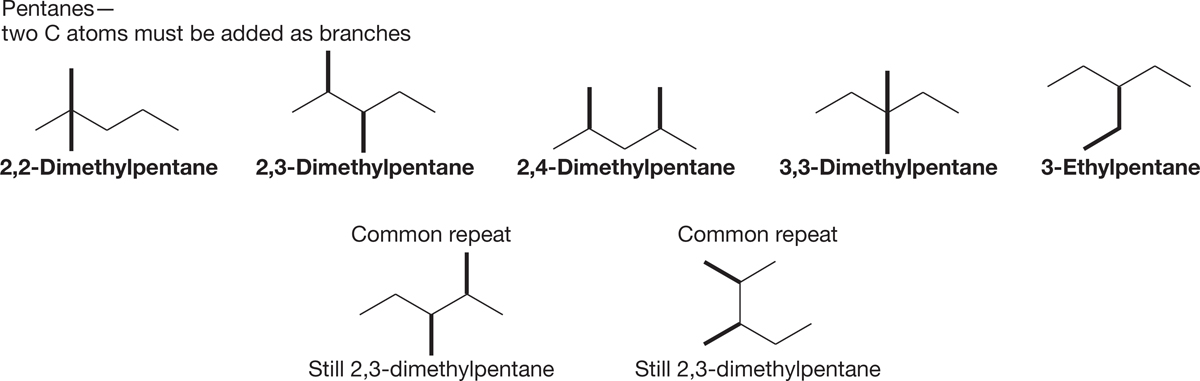
FIGURE 2.47 3-Ethylpentane and the dimethylpentanes.

FIGURE 2.48 Trimethylbutane.
PROBLEM SOLVING
Draw all the isomers having the molecular formula C7H16.
1. Start by drawing the longest unbranched carbon chain possible, which is heptane in this case (Fig. 2.45).
2. Shorten the chain by one carbon, and add the “extra” carbon as a methyl group at all possible positions, starting at the left and moving to the right (Fig. 2.46). Note that you can’t add this carbon to the end of the chain because doing that would just regenerate heptane. You have to start the addition process one carbon in from the end. This step generates all the methylhexanes. You must check each isomer you make to be sure it is not a repeat. One way to be absolutely certain is to name each molecule as you generate it. If you repeat a name, you have repeated an isomer.
3. Shorten the chain by one more carbon. The longest straight chain is now five carbons. Two carbons are to be added as two methyl groups or as a single ethyl group (Fig. 2.47). Two methyl groups can be placed either on the same carbon or on different carbons. Again, start at the carbon one in from the left end of the chain and move to the right, checking each isomer you create by naming it to be sure it is not a repeat.
4. Shorten the chain by one carbon again, and try to fit the three “extra” carbons on a butane chain. There is only one way to do this (Fig. 2.48).
PROBLEM 2.28 Write and name all the isomers of octane, C8H18.