2.3 The Methyl Group (CH3) and Methyl Compounds (CH3X)
As soon as one of the four hydrogens surrounding the central carbon in methane is replaced with another atom, pure tetrahedral symmetry is lost. We might well anticipate that one of the four sp3 hybrid orbitals of carbon could overlap in a stabilizing way with any atom X offering an electron in any atomic orbital (Fig. 2.11). For maximum stabilization, we want to fill the new, bonding molecular orbital created from this overlap, which requires two, and only two, electrons.
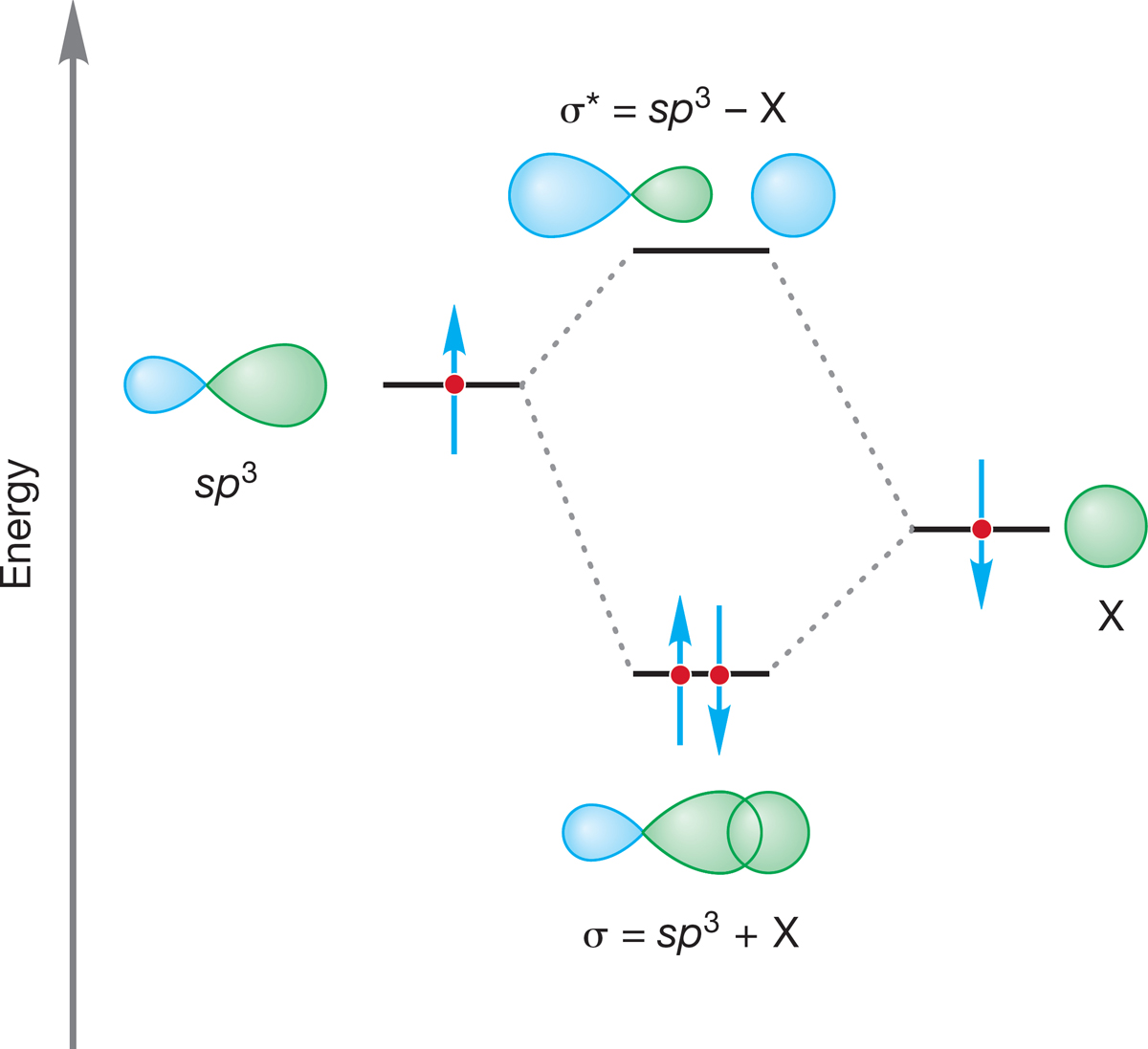
FIGURE 2.11 Formation of a C—X bond from overlap of an sp3 hybrid orbital of C with an s atomic orbital of X.
All manner of derivatives of methane, called methyl compounds (CH3―H), are possible. The atom or group replacing H is called a substituent, and one option for naming these substituted compounds is to drop the “ane” from the name of the parent molecule, methane, and append “yl.” The methyl is followed by the name of the X group as a separate word. Table 2.2 shows several methyl derivatives with their melting points, boiling points, and physical appearances.
The bonding in methyl compounds closely resembles that in methane, and it is conventional to speak of the carbon atom in any H3C―X as being sp3 hybridized, just as it is in the more symmetrical CH4. Strictly speaking, this is wrong because the bond from C to X is not the same length as that from C to H, and the H―C―H bond angle cannot be exactly the same as the X―C―H angle. Because sp3 hybridization yields four exactly equivalent bonds directed toward the corners of a tetrahedron, the bonds to H and X in H3C―X cannot be exactly sp3 hybrids, only approximately sp3 (sp2.8, say). This point is very often troubling to students, but it is really quite simple. Consider converting an sp2-hybridized carbon into an sp3-hybridized carbon, as in Figure 2.12. We start with the carbon having three sp2 hybrid orbitals and a pure, unhybridized 2p orbital (H―C―H angle = 120°). As we bend the sp2 hybrid orbitals, we come to a point at which the H―C―H angle has contracted to 109.5°, which is sp3 hybridization. Our orbitals, originally one-third s and two-thirds p (sp2: 33.3% s, 66.7% p character), have become one-fourth s and three-fourths p (sp3: 25% s, 75% p character). They have gained p character in the transformation from sp2 to sp3. Now look at the 2p atomic orbital in Figure 2.12. As we bend the hybrid orbitals, this orbital goes from pure p to one-fourth s, three-fourths p. It has gained s character in the transformation. There is a smooth transformation as the orbitals change from sp2 to sp3, and between these two extremes, the hybridization of carbon is intermediate between sp2 and sp3, something like sp2.8. The hybridizations sp2 and sp3 are limiting cases, applicable only in completely symmetrical situations. The hybridization of a carbon (or other) atom is intimately related to the bond angles. If you know one, you know the other.
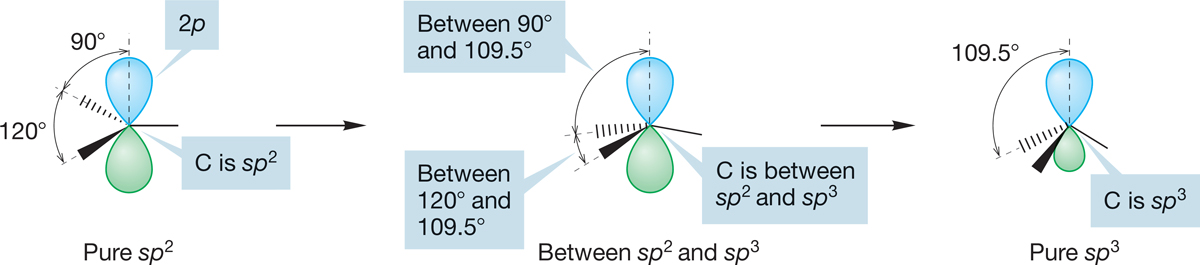
FIGURE 2.12 A thought experiment. The conversion of an sp2-hybridized carbon into an sp3-hybridized carbon. What is the hybridization at a point intermediate between the starting point (sp2) and end point (sp3)?
TABLE 2.2 Some Simple Derivatives of Methane, Otherwise Known as Methyl Compounds
H3C―X |
Common Name |
mp (°C) |
bp (°C) |
Physical Properties |
|
H3C―H |
Methane |
−182.5 |
−164 |
Colorless gas |
|
H3C―OH |
Methyl alcohol, or methanol |
−93.9 |
65.0 |
Colorless liquid |
|
H3C―NH2 |
Methylamine |
−93.5 |
−6.3 |
Colorless gas |
|
H3C―Br |
Methyl bromide |
−93.6 |
3.6 |
Colorless gas/liquid |
|
H3C―Cl |
Methyl chloride |
−97.7 |
−24.2 |
Colorless gas |
|
H3C―CN |
Methyl cyanide, or acetonitrile |
−45.7 |
81.6 |
Colorless liquid |
|
H3C―F |
Methyl fluoride |
−141.8 |
−78.4 |
Colorless gas |
|
H3C―I |
Methyl iodide |
−66.5 |
42.4 |
Colorless liquid |
|
H3C―SH |
Methyl mercaptan, or methanethiol |
−123 |
6.2 |
Colorless gas/liquid |
|
mp, melting point; bp, boiling point.
In general, methyl compounds (H3C―X) closely resemble methane in their approximately tetrahedral geometry: sp3 is a good approximation, but methyl compounds cannot be perfect tetrahedra.
In addition to CH3 X, other substituted molecules are possible in which more than one hydrogen is replaced by another atom or group of atoms. Thus, even for one-carbon molecules, there are many possible substituted structures.
PROBLEM 2.9 Use the halogens (X = F, Cl, Br, or I) to draw all possible molecules CH2X2. For example, CH2BrCl is one answer.
PROBLEM 2.10 Draw all possible molecules of the formula CH2X2, CHX3, and CX4 when X is F or Cl.