2.7 Propane (C3H8) and Propyl Compounds (C3H7X)
Propane, the third member of the alkane series, contains three carbon atoms and has many of the chemical and physical properties of ethane and methane. Figure 2.27 shows several representations for propane, including two new ones. In Figure 2.27d, only the carbon frame is retained, and all eight hydrogen atoms are implied. Not even the carbons are shown in Figure 2.27e, and the reader is left to fill in mentally all the atoms. This most abstract representation—called a line drawing of the molecule—is the representation of choice for organic chemists, and we will gradually slip into this way of drawing molecules in subsequent chapters. In a line drawing of a hydrocarbon, every angle vertex and terminus is a carbon, never a hydrogen, and you have to add hydrogens to every carbon so that carbon’s valence of four bonds is satisfied.
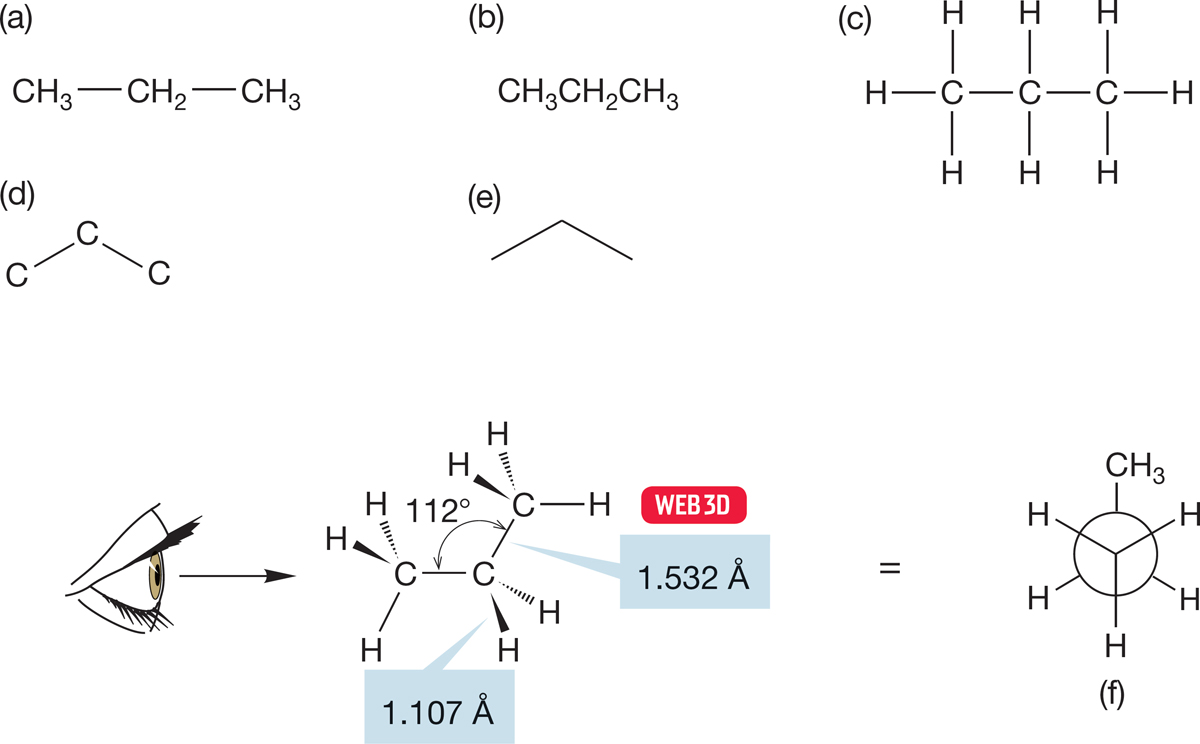
FIGURE 2.27 Different representations of propane.
Notice that some but not all of the schematic representations for propane show that it is not a strictly linear species. All carbons are hybridized approximately sp3, and the C―C―C angle is close to 109° (in fact, it is 112°).
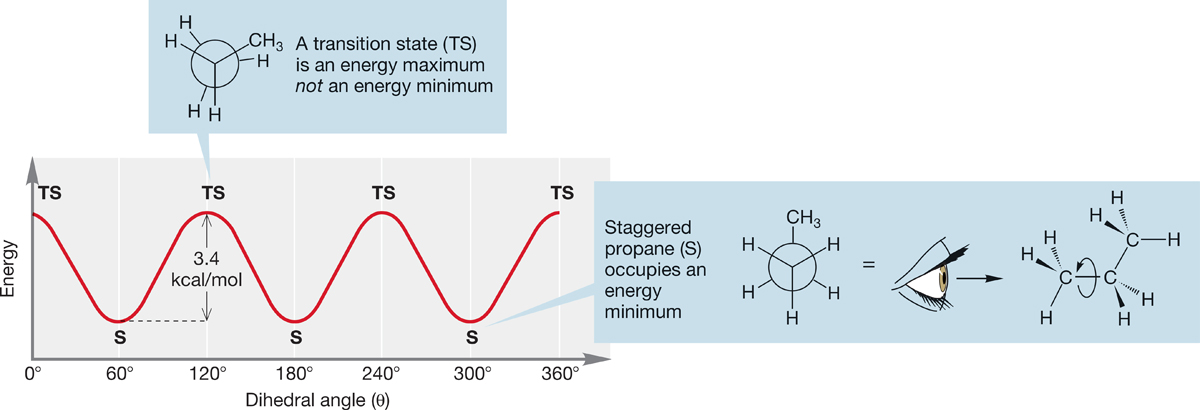
FIGURE 2.28 A graph of energy versus dihedral angle (θ) for propane.
The Newman projection for propane (Figs. 2.27f and 2.28), constructed by looking down one of the two equivalent carbon–carbon bonds, shows the structure of propane particularly well. A plot of energy versus dihedral angle for propane, shown in Figure 2.28, is similar to that for ethane, except that the rotational barrier of 3.4 kcal/mol (14.2 kJ/mol) is slightly higher than that of ethane. In propane, a large methyl group is eclipsed with a hydrogen at the top of the barrier (the transition state), and this spatial interaction accounts for the higher energy.
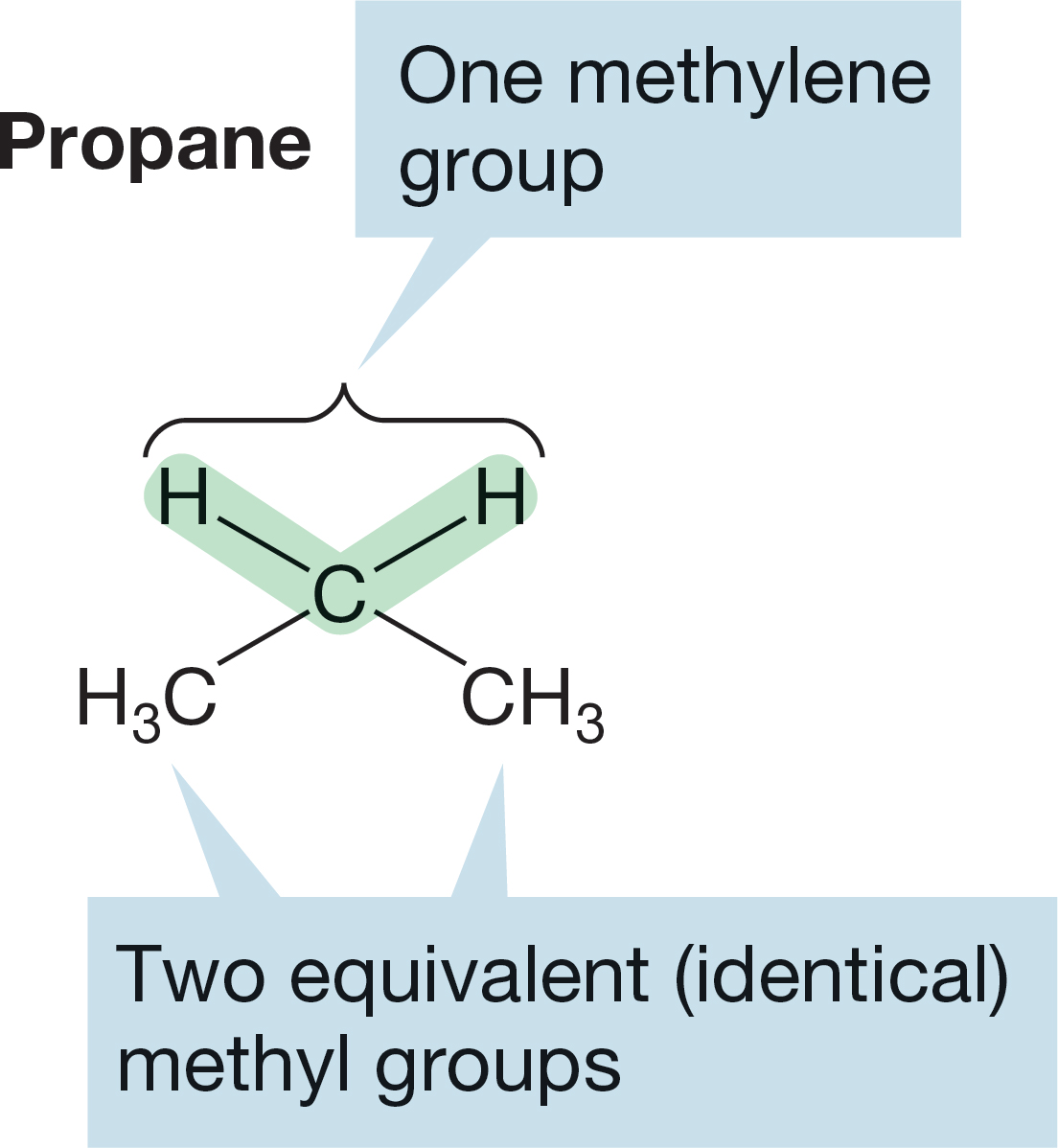
FIGURE 2.29 The two types of hydrogen in propane.
Replacement of a hydrogen in propane with an X group yields propyl derivatives, but the situation is more complicated than in methane or ethane. In both methane and ethane, all hydrogens are equivalent, and there can be only one methyl or one ethyl derivative. However, propane has two different kinds of hydrogen. There is a central CH2 group, called a methylene group, and two equivalent methyl groups at the two ends of the molecule (Fig. 2.29). The carbon and hydrogens in a methylene group (CH2) are not the same as the carbon and hydrogens in a methyl group (CH3).
Either of these two kinds of hydrogen can be replaced by an X to give a propyl compound (Fig. 2.30). The linear compound CH3―CH2―CH2—X, in which X replaces a methyl hydrogen, is called propyl X (in the older literature, n-propyl X). The branched compound CH3―CHX―CH3, in which X replaces a hydrogen on the methylene carbon, is called isopropyl X (and sometimes i-propyl X). Propyl compounds and isopropyl compounds are structural (constitutional) isomers of each other; they are compounds with the same formula but different structures.
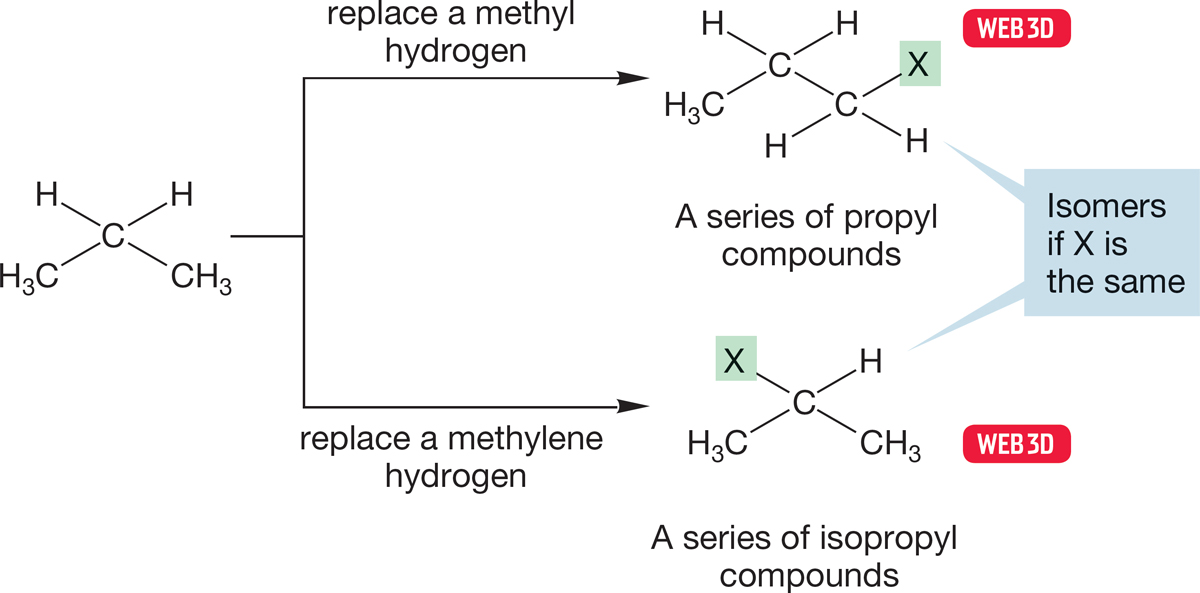
FIGURE 2.30 Propyl and isopropyl compounds.
PROBLEM 2.20 Make a three-dimensional drawing of propyl alcohol (CH3―CH2―CH2―OH) and one of the related isopropyl alcohol (CH3―CHOH―CH3).