6.8 Special Topic: Sulfur Compounds
Thiols (RSH) and thioethers (RSR′) are the sulfur-containing counterparts of alcohols and ethers, and their chemistries are generally similar to those of their oxygen-containing relatives. These sulfur compounds have a poor reputation because some are quite extraordinarily evil-smelling. The skunk uses a variety of four-carbon thiols in its defense mechanism, for example. But this bad press is not entirely deserved; not all sulfur compounds are malodorous. Garlic derives its odor from small sulfur-containing compounds. You may never need to ward off a vampire with it, but garlic contains many sulfur compounds that have quite remarkably positive qualities. Figure 6.60 shows some of the sulfur-containing molecules that can be found in garlic.
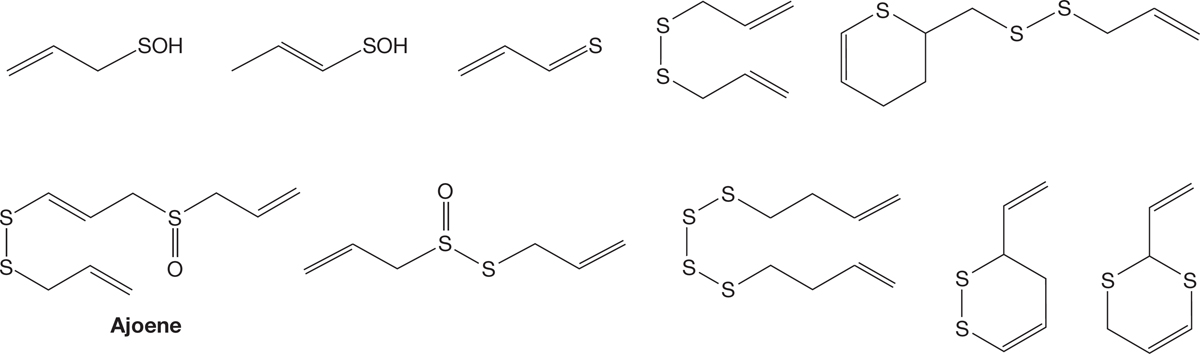
FIGURE 6.60 Some of the sulfur-containing molecules in garlic.
AJOENE
Ajoene is only one of the beneficial compounds present in garlic. Ajoene is lethal to certain tumor-prone cells, promotes the antiaggregative action of some molecules on human blood platelets, and seems to be effective against viruses (including HIV) as well. It appears to reduce repeat heart attacks among people who have already suffered an initial attack. Garlic contains many other compounds that also appear to have beneficial medicinal properties. And, of course, if that vampire is hot on your trail. . . .

Garlic is good for the diet and protects from vampires.
6.8a Nomenclature Thiols, also called mercaptans, are named by adding the suffix “thiol” to the parent hydrocarbon name. Note that the final “e” is not dropped as it is, for example, in alcohol nomenclature (Fig. 6.61).
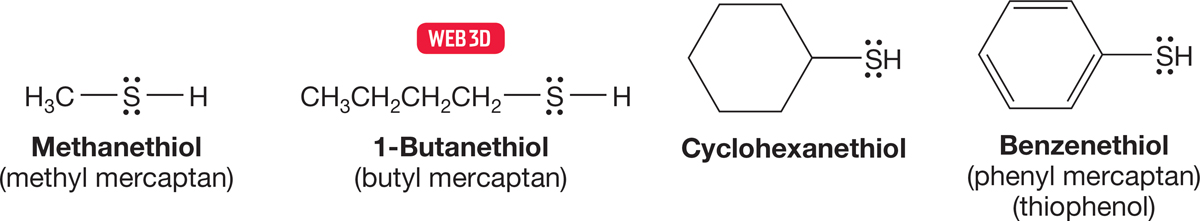
FIGURE 6.61 Some thiols, or mercaptans.
Thioethers, the sulfur counterparts of ethers, are also called sulfides and are named in the same way as ethers. The two groups attached to the sulfur atom are followed by the word sulfide. Disulfides (R―S―S―R) are the counterparts of peroxides (R―O―O―R), and have a significant biological role, which we will discuss in Chapter 16. Peroxides are nastily unstable and tend to explode, but disulfides are relatively benign. Disulfides are named in a fashion similar to that for sulfides (Fig. 6.62).
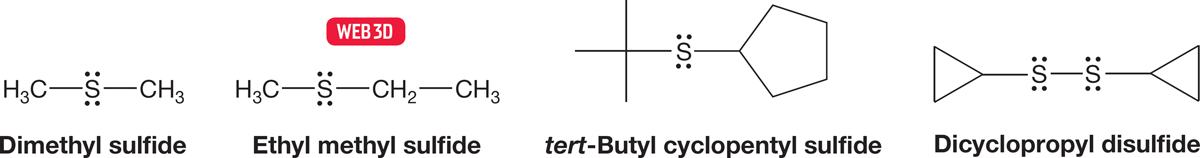
FIGURE 6.62 Some thioethers (sulfides) and disulfides.
6.8b Acidity Thiols (pKa = 9 to 12) are stronger acids than alcohols and form mercaptides, the sulfur counterparts of alkoxides, when treated with base. The relatively high acidity of thiols makes formation of the conjugate base more favorable than formation of alkoxides from alcohols (Fig. 6.63).
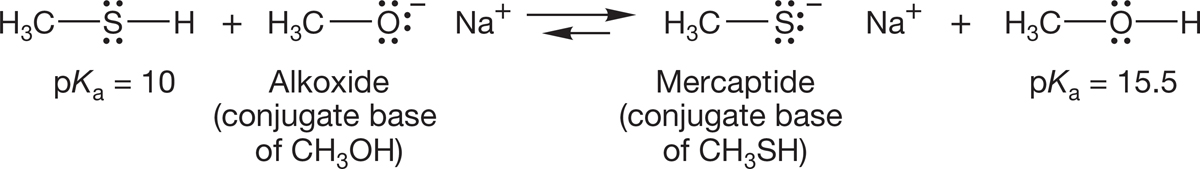
FIGURE 6.63 Mercaptides are easier to form than alkoxides because thiols are much more acidic than alcohols.
6.8c Reduction of Sulfur Compounds with Raney Nickel: A New Alkane Synthesis Thiols and thioethers are reduced by a catalyst called Raney nickel (essentially just hydrogen adsorbed on finely divided nickel) to give hydrocarbons (Fig. 6.64). This synthetic method, called desulfuration, gives you a second way to make alkanes.
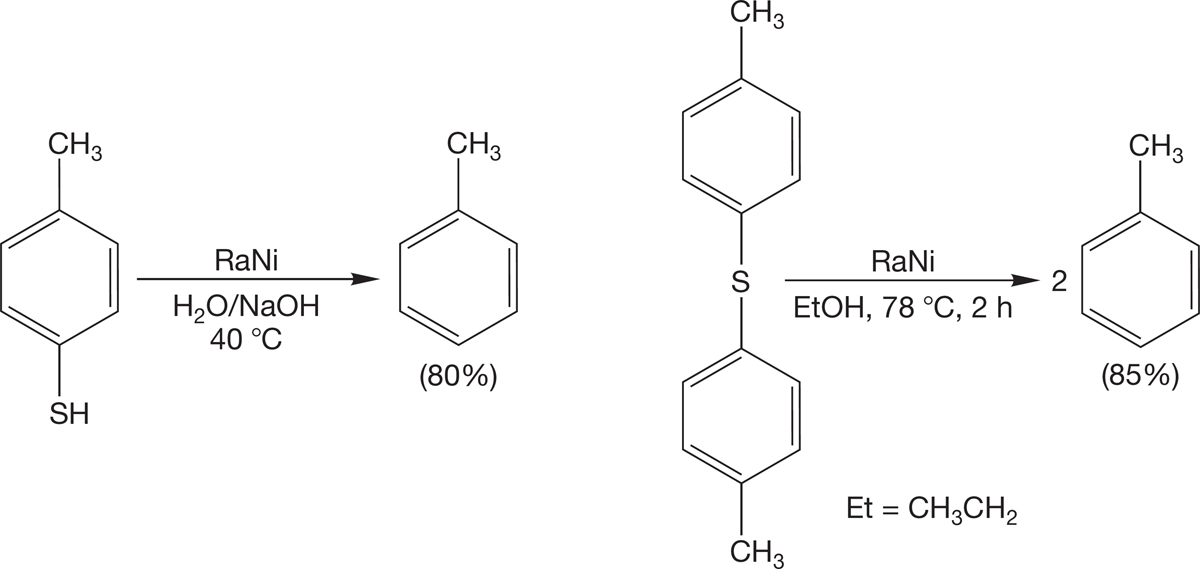
FIGURE 6.64 Desulfuration with Raney nickel (RaNi).
PROBLEM 6.20 What reagents would you use to convert the indicated starting materials into cyclohexane?