2.1 Preview
The chemical reactions a given compound undergoes are a function of its structure. No compound reacts exactly like another, no matter how similar their structures. However, there are gross generalizations that can be made, and some order can be carved out of the chaos that would result if compounds of similar structure did not react in somewhat the same fashion. As we will see in this chapter, to a first approximation, the reactivity of a molecule depends on the functional groups it contains. A molecule can have more than one functional group, in which case the molecule will have multiple types of reactivity.
The inside front cover of this book shows the functional groups that are most important in organic chemistry. You will want to work through the list to be certain you can write correct Lewis structures for each group and to begin familiarizing yourself with the variety of functional groups important in organic chemistry. These different kinds of molecules will all be discussed in detail in later chapters. We start in this chapter with alkanes, the simplest members of the family of organic molecules called hydrocarbons—compounds composed entirely of carbon and hydrogen.
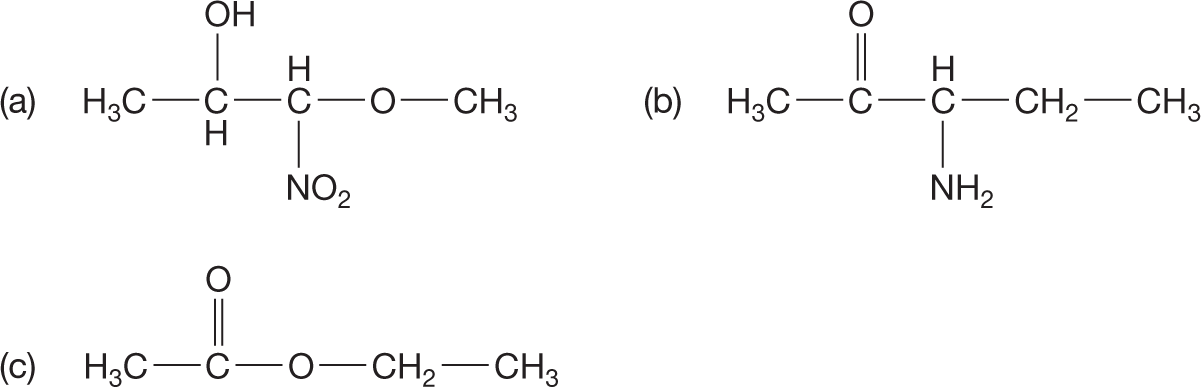
PROBLEM 2.1 Circle and name the functional groups in the following molecules. See the inside front cover of the book.
Alkanes all have the molecular formula CnH2n+2. The simplest alkane is methane, CH4. Vast numbers of related molecules can be constructed from methane by replacing one or more of its hydrogens with other carbons and their attendant hydrogens. Linear arrays can be made, as well as branched structures and even rings (hydrocarbon ring compounds called cycloalkanes have a slightly different formula: CnH2n). Figure 2.1 shows a few schematic representations for these molecules. Before we can go further, we need to make sense of these schematics, and as usual, we must start with structure. The first task is to describe the shape of methane. Once we have done this, we can go on to investigate other members of the alkane family.
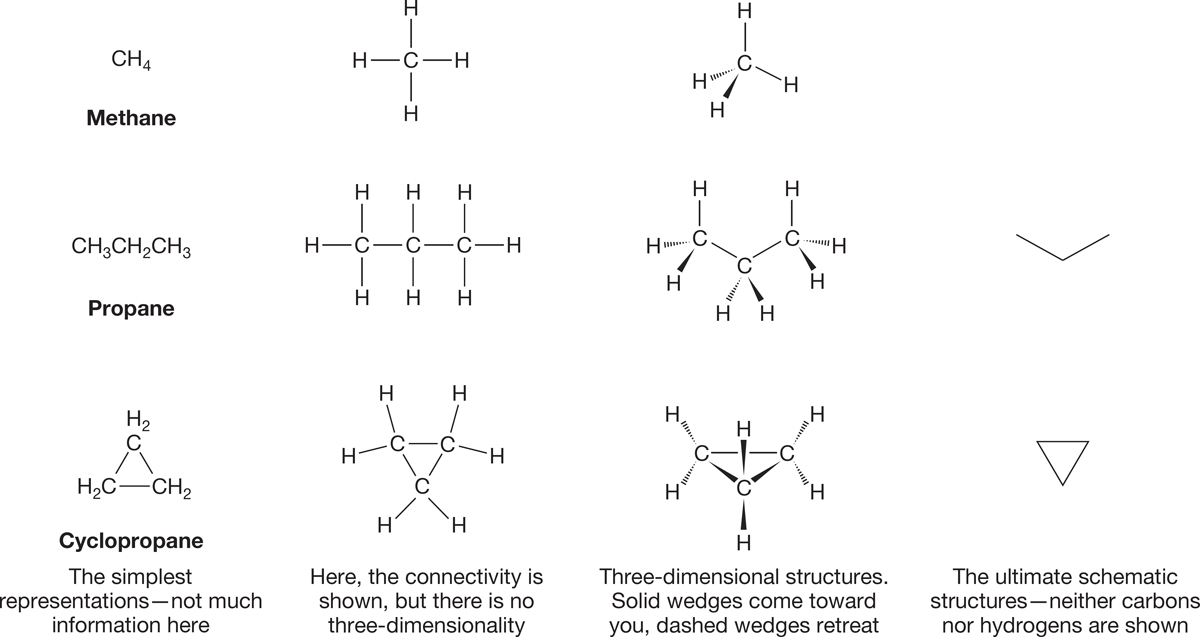
FIGURE 2.1 Several ways of drawing alkane structures.
ESSENTIAL SKILLS AND DETAILS
1. Hybridization. Above all, it is important to master the structural model introduced in this chapter: hybridization. The hybridization model does a good job of allowing us to predict the general structures of compounds, and it is nicely suited for following electron flow in chemical reactions. Thus, it fits in well with the curved arrow formalism introduced in Chapter 1 (p. 24).
2. Structures. It is important to be able to use the various structural formulas, which range from richly detailed three-dimensional representations to the ambiguous condensed formulas that give no hint of the three-dimensional complexity often present in a molecule.
3. Difference. The concept of difference is essential in organic chemistry. When are two atoms the same (in exactly the same environment) and when are they different (not in the same environment)? This question gets to the heart of structure and is much tougher to answer than it seems. In this chapter, we will introduce this subject, and we will return to it in Chapter 4.
4. Names. In truth, it is not necessary to know every nuanced detail of the naming convention for alkanes, but you do need to know some nomenclature.
5. The cis/trans convention. Cyclic molecules (rings) have sides—above the ring and below the ring—and attached groups can be on the same or opposite sides of the ring.
6. The Newman projection. Drawing and “seeing” Newman projections is a critical skill in organic chemistry.