1
Atoms and Molecules; Orbitals and Bonding
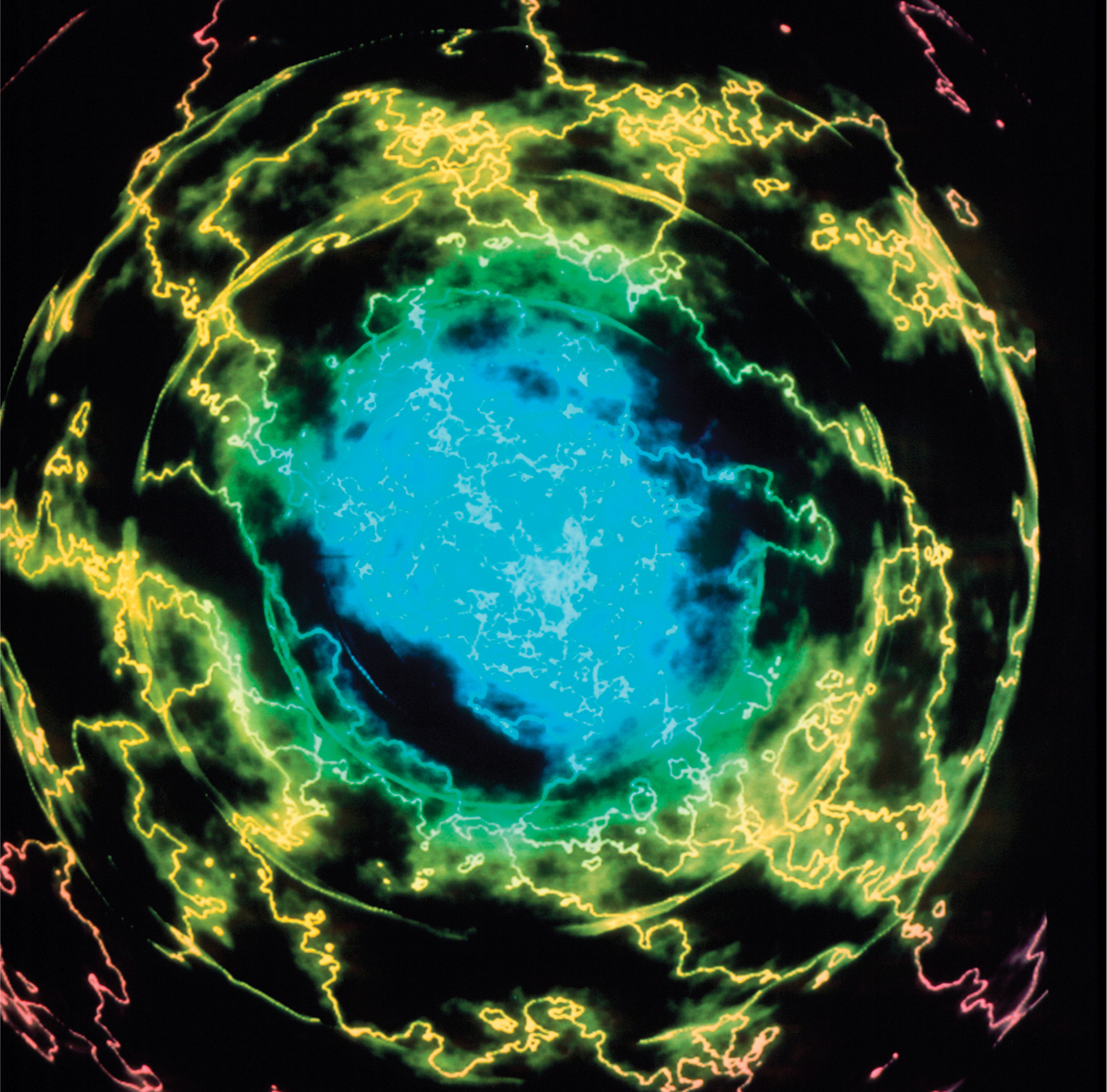
ELECTRON DENSITY OF A HELIUM ATOM This image represents the quantum cloud of electrons surrounding a helium atom. Colors represent the local electric charge density, essentially the probability that one of the atom’s four electrons will be found at a given point. The colors run from blue (highest probability) to red (lowest probability). The chaotic appearance of the image is due to the presence of a nearby charged particle; when in equilibrium, the atom would appear more symmetrical. This is a more appropriate illustration than the older ideas of fixed “orbitals” for electrons, sometimes referred to as the Bohr model, and is due to the principles of modern quantum theory.
When it comes to atoms, language can be used only as in poetry. The poet too, is not nearly so concerned with describing facts as with creating images.
—NIELS BOHR TO WERNER HEISENBERG1
1Niels Bohr (1885–1962) and Werner Heisenberg (1901–1976) were pioneers in the development of quantum theory, the foundation of our current understanding of chemical bonding.