1.8 An Introduction to Reactivity: Acids and Bases
Even at this early point, we can begin to consider what many see as the real business of chemistry—reactivity. The orbital interaction diagrams we have just learned to draw lead us to powerful unifying generalizations. Remember that we can fit no more than two electrons into any atomic or molecular orbital (p. 8). There are two ways to provide the electrons that fill the bonding molecular orbital so as to stabilize two electrons. Figure 1.49a shows a pair of electrons, each in a singly occupied orbital, combining to produce a bond in which the two electrons are stabilized (both electrons move down to a lower energy value). Alternatively, we could imagine the situation outlined in Figure 1.49b, in which a filled orbital mixes with an empty orbital to produce a similar stabilization. An atom or molecule that is a two-electron donor is called a Lewis base. An atom or molecule that is a two-electron acceptor is called a Lewis acid. These labels were introduced by G. N. Lewis (p. 13).
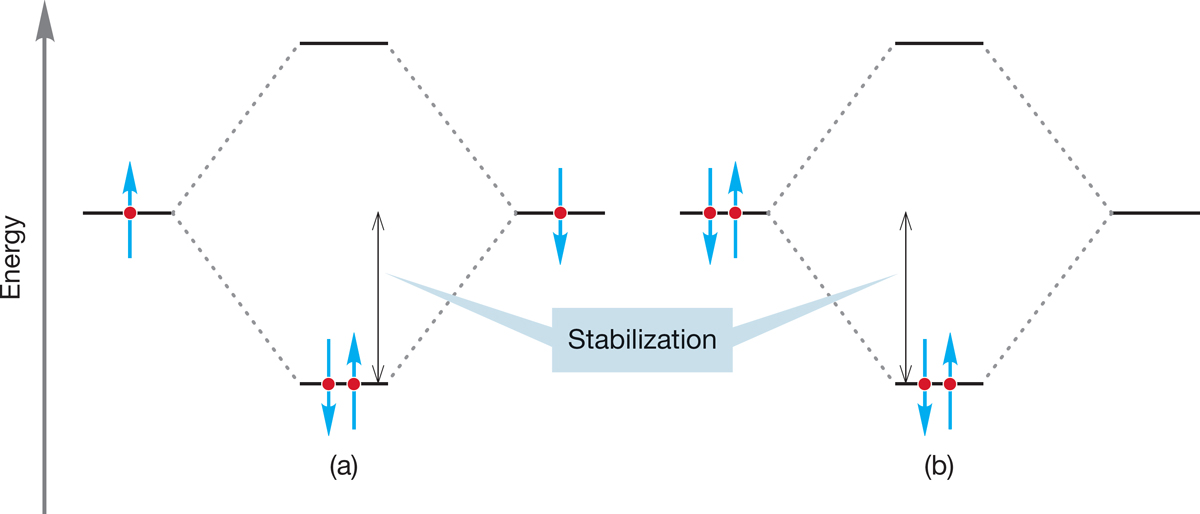
FIGURE 1.49 Two interactions that lead to stabilization of a pair of electrons.
Both of these bond-forming scenarios are common, but the scenario in Figure 1.49b is especially important. We have already seen an example of the process shown in Figure 1.49a when we considered a pair of hydrogen atoms combining to form H2 (Fig. 1.44). We have not yet seen any real examples of the Lewis acid–Lewis base situation, but we will encounter hundreds as we work through this book.
You are probably familiar with Brønsted acids and bases (proton donors and acceptors, respectively), but the situation described by Figure 1.49b is much more general. It would seem that a stabilizing (energy-lowering) bond-forming interaction can take place whenever a filled orbital overlaps with an empty orbital. We will give this important idea the careful treatment it deserves in future chapters, but it is worth thinking about it a bit right now.
Organic chemists use the terms electrophile for a Lewis acid and nucleophile for a Lewis base. The combination of a Lewis base and a Lewis acid (or nucleophile plus electrophile), conceptualized in the orbital interaction diagram of Figure 1.49b, leads to formation of a covalent bond. The concepts that “a nucleophile reacts with an electrophile” and “the interaction of a filled orbital with an empty orbital leads to bond formation and stabilization of two electrons” run throughout organic (and other) chemistry. These two concepts allow us to generalize and not be swamped by all the prolific detail soon to come. Nucleophile plus electrophile is the unifying theme of organic chemistry.
PROBLEM 1.33 Identify the Lewis base (nucleophile) and Lewis acid (electrophile) in each of these reactions. Write the molecule formed in each case.
(a) H+ H:− → ?
(b) 
(c) H3C+ −:CH3 → ?
(d) H3C+ :NH3 → ?
PROBLEM 1.34 Here is a chance to practice your electron pushing. Draw curved arrow formalisms for all the reactions in Problem 1.33.