2.6 Structure Drawings
CONVENTION ALERT
Methyl and Ethyl Abbreviations
In the preceding sections, we presented many different representations of the very simple molecule ethane. Figure 2.26 recapitulates them and adds some new ones. Methyl groups are sometimes written as Me and ethyl groups as Et, especially in colloquial use.
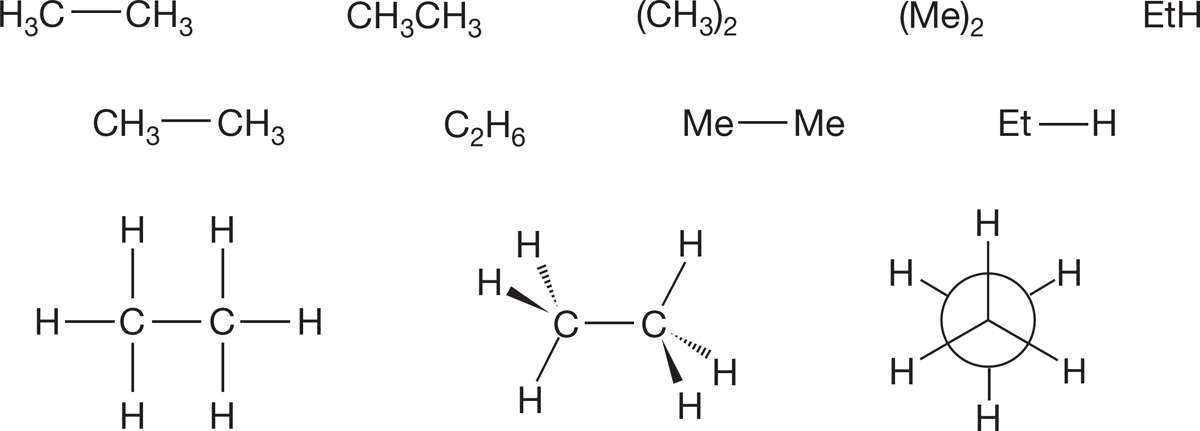
FIGURE 2.26 Twelve different representations of ethane.
The difficulty of representing the real, dynamic, three-dimensional structure of ethane should be more apparent to you now. In the real world, we often do not have the time to draw out carefully a good representation of even as simple a molecule as ethane. The solid and dashed wedges of Figure 2.26 are the traditional attempts at adding a three-dimensional feel to the two-dimensional drawing. More complicated molecules can raise horrendous problems. Adequate codes are needed, and you must learn to see past the coded structures to the real molecules both easily and quickly. It’s worth considering here, at this very early point, some of the pitfalls of the various schemes.
First of all, it is not simple to represent even ethane effectively in a linear fashion. Clearly, neither C2H6 nor Et―H is as descriptive as H3C―CH3, as those formulas don’t show the connectivity of the atoms in a proper way. The representation H3C―CH3 is better, but even this picture lacks three dimensionality. We used this representation in this chapter, but you will sometimes see the variant CH3―CH3. The two formulations are equivalent, even though the CH3―CH3 seems to indicate that the bond to the right-hand carbon comes from one of the hydrogens on the left-hand carbon. Of course, it does not; it is the two carbons that are bonded, as H3C―CH3 shows. Yet it is easier to write CH3―CH3, and you will see this form often.
PROBLEM 2.18 Draw three-dimensional structures for (Me)2CH2, (CH3)4C, (CH3)3CH, EtCH3, (Et)2, CH3CH2CH3, EtMe, and MeEt.
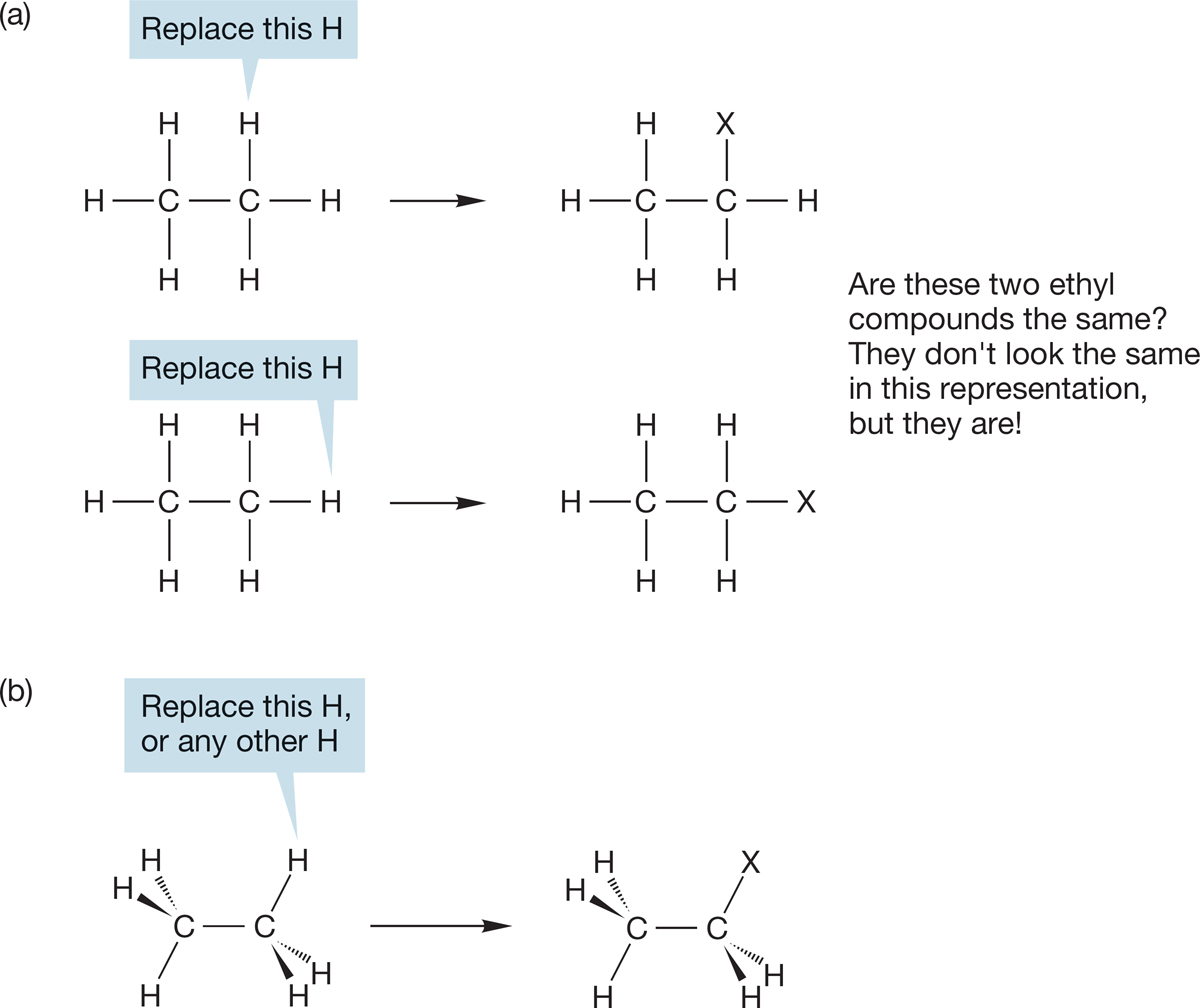
PROBLEM 2.19 Start with the two “different” structures in Figure 2.25a and replace the X group with CH3 in each. Make three-dimensional drawings of the “two” molecules you’ve created and convince yourself that both your three-dimensional drawings represent the same molecule; there is only one CH3CH2CH3. By all means, use your models.