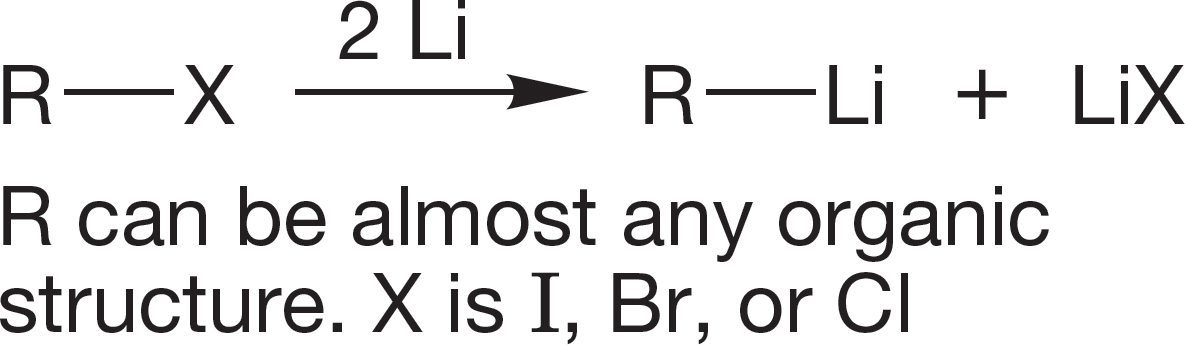6.10 Summary
New Concepts
A measure of a compound’s acidity—its pKa value—is introduced. The lower the pKa value of a compound, the more acidic it is.
The solvent is an important factor in stabilizing ions. For example, both the conjugate bases of alcohols (alkoxides) and the conjugate acids of amines (ammonium ions) depend greatly on solvation for stabilization. In assessing basicity or acidity, you must take account of the solvent.
A general discussion of solvents and solvation also appears. Protic solvents that can participate in hydrogen bonding dissolve other protic, polar molecules well. By contrast, polar solvents do a poor job of dissolving “greasy” nonpolar organic molecules such as hydrocarbons, which are dissolved by nonpolar, similarly “greasy” solvents. Like dissolves like—polar solvents dissolve polar molecules, and nonpolar solvents dissolve nonpolar molecules.
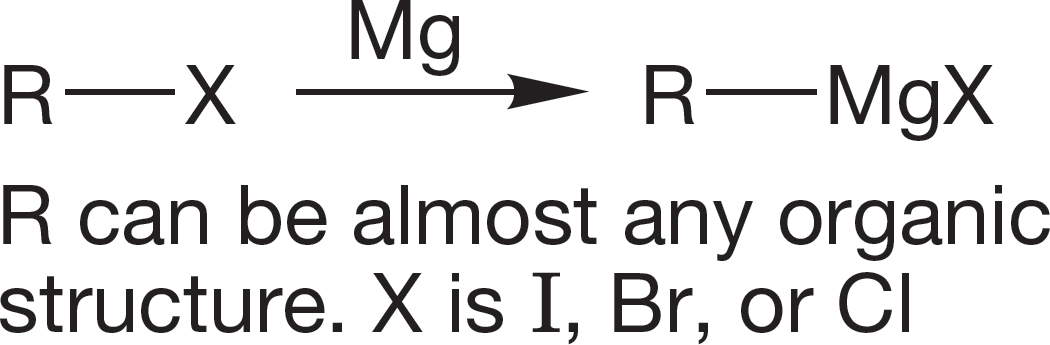
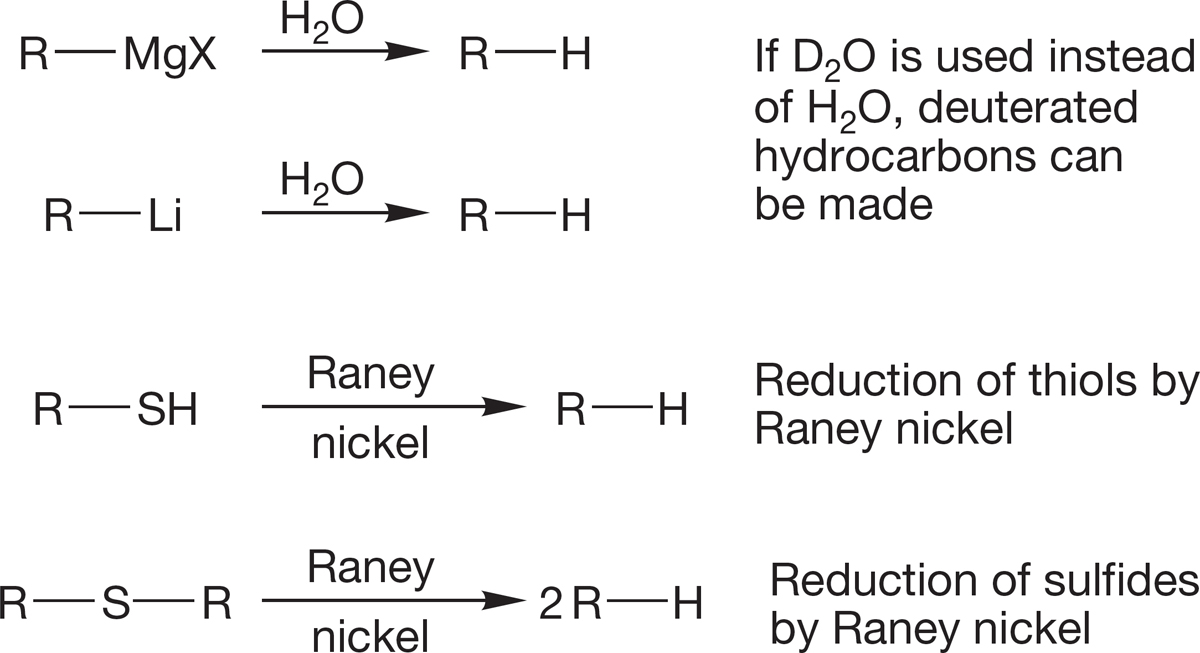
Organic halogenated molecules can be used to form organometallic reagents. Many of the important synthetic reactions of organic chemistry use organometallic reagents. In this chapter, we see them only as very strong bases and as sources of hydrocarbons through their reaction with water.
Amines, like alkanes with sp3-hybridized carbons, are tetrahedral. Unlike alkanes, amines invert their “umbrellas” through an sp2-hybridized transition state.
We also introduced the hydrogen bond. This kind of bond is nothing more than a partially completed acid–base reaction in which a partial bond is formed between a pair of electrons on one atom and a hydrogen on another (Fig. 6.68).
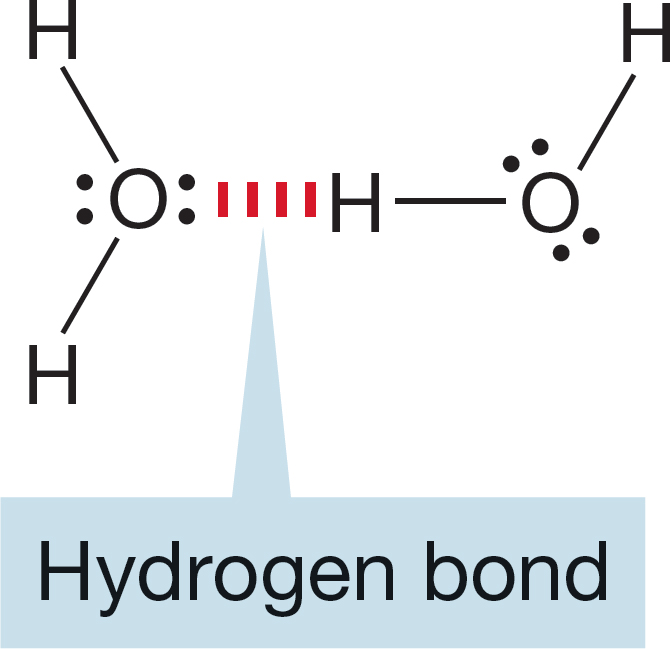
FIGURE 6.68 A hydrogen bond.
Key Terms
alkoxide ion
alkyl halide
amide
amine
amine inversion
ammonia
ammonium ion
aprotic solvent
aziridine
conjugate acid
conjugate base
crown ether
cryptand
diol
epoxide
ether
free radical
glycol
Grignard reagent
hydrogen bonding
mercaptan
mercaptide
organolithium reagent
organometallic reagent
oxirane
peptide
pKa
primary amine
protein
protic solvent
Raney nickel
secondary amine
solvation
sulfide
tertiary amine
thioether
thiol
Common Errors
People sometimes get the connection between pKa and acid strength wrong. A strong acid has a low pKa; a weak acid has a high pKa.
It is easy to get confused when using the pKa of an ammonium ion as a measure of the basicity of the related amine.
Review the discussion (p. 250) or use the more direct (but less common) pKb values.
Syntheses
1. Grignard Reagents
2. Hydrocarbons
3. Organolithium Reagents