6.4 Properties of Substituted Alkanes
6.4a Polarity Substituted alkanes are polar molecules. Halogens are relatively electronegative atoms and strongly attract the electrons in the carbon–halogen bond of alkyl halides, which have dipole moments of approximately 2 D (Fig. 6.23; Table 6.2).
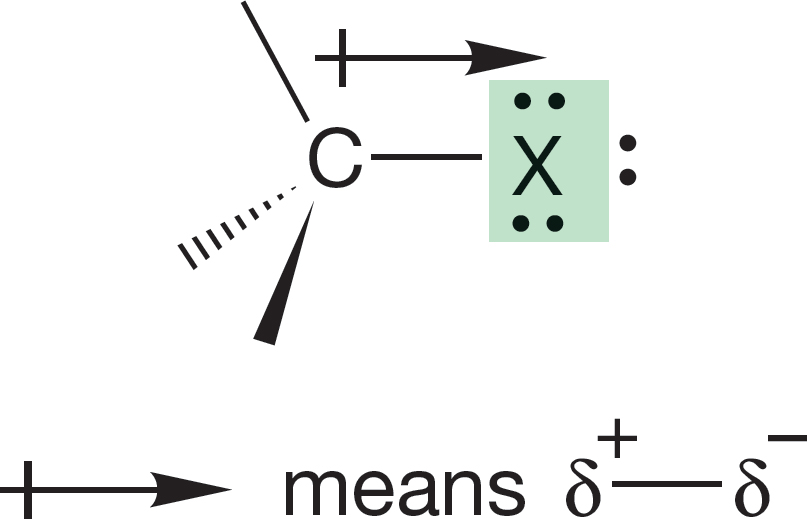
FIGURE 6.23 A substantial dipole moment exists in the polar carbon–halogen bond, X = F, Cl, Br, or I.
These polar molecules can associate in solution, and therefore the boiling points tend to be higher than those of their hydrocarbon counterparts (compare Tables 2.4 and 6.1).
TABLE 6.2 Dipole Moments of Some Simple Alkyl Halides
Alkyl Halide |
Dipole Moment (D) |
Methyl fluoride |
1.85 |
Ethyl fluoride |
1.94 |
Methyl chloride |
1.87 |
Ethyl chloride |
2.05 |
Methyl bromide |
1.81 |
Ethyl bromide |
2.03 |
Methyl iodide |
1.62 |
Ethyl iodide |
1.91 |
|
|
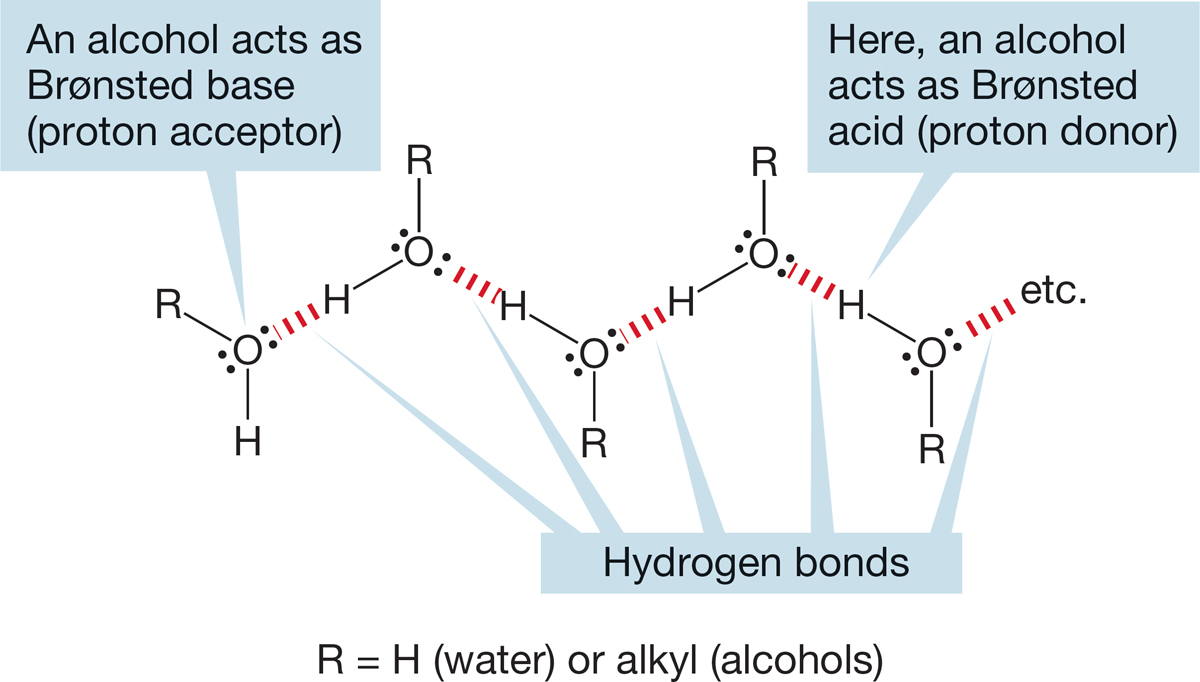
FIGURE 6.24 Water and alcohols are both Brønsted acids and Brønsted bases. This figure shows hydrogen bonding between molecules of water (R = H) and alcohols.
In alcohols (ROH), the presence of the electronegative oxygen atom ensures that bonds are strongly polarized and that substantial dipole moments exist. In the liquid phase, alcohols are strongly associated, both because of dipole–dipole attractions and because of hydrogen bonding, in which the basic oxygen atoms form partial bonds to the acidic hydroxyl hydrogens. As you probably remember from general chemistry, hydrogen bonding is the noncovalent interaction between a hydrogen that is covalently attached to an oxygen (or nitrogen or fluorine) and another oxygen (or nitrogen or fluorine) to which it is not covalently attached. Such interactions can be quite strong, approximately 5 kcal/mol (21 kJ/mol), a value not high enough to make permanent dimeric or polymeric structures but quite sufficient to raise the boiling points of alcohols far above those of the much less strongly associated alkanes. Figure 6.24 shows an ordered, hydrogen-bonded structure for an alcohol. In the absence of hydrogen bonding, water (with a molecular weight of only 18) and the low molecular weight alcohols would surely be gases. The polarity of alcohols makes them quite water soluble, and most of the smaller molecules are miscible (soluble in all proportions) with, or at least highly soluble in, water. Table 6.3 gives some physical properties of alcohols. For comparisons with the parent alkanes, see Table 2.4 (p. 83).
TABLE 6.3 Some Physical Properties of Alcohols
Compound |
bp (ºC) |
mp (ºC) |
Density (g/mL) |
Dipole Moment (D) |
CH3OH |
65.15 |
–93.9 |
0.79 |
1.7 |
CH3CH2OH |
78.5 |
–117.3 |
0.79 |
1.69 |
(CH3)2CHOH |
82.4 |
–89.5 |
0.80 |
1.68 |
CH3CH2CH2CH2OH |
117.2 |
–89.5 |
0.81 |
1.66 |
(CH3)2CHCH2OH |
108 |
–108 |
0.80 |
1.64 |
CH3CH2CH(CH3)OH |
99.5 |
–115 |
0.81 |
1.7 |
(CH3)3COH |
82.3 |
25.5 |
0.79 |
1.7 |
|
181.7 |
43 |
1.06 |
1.45 |
|
||||
bp, boiling point; mp, melting point.
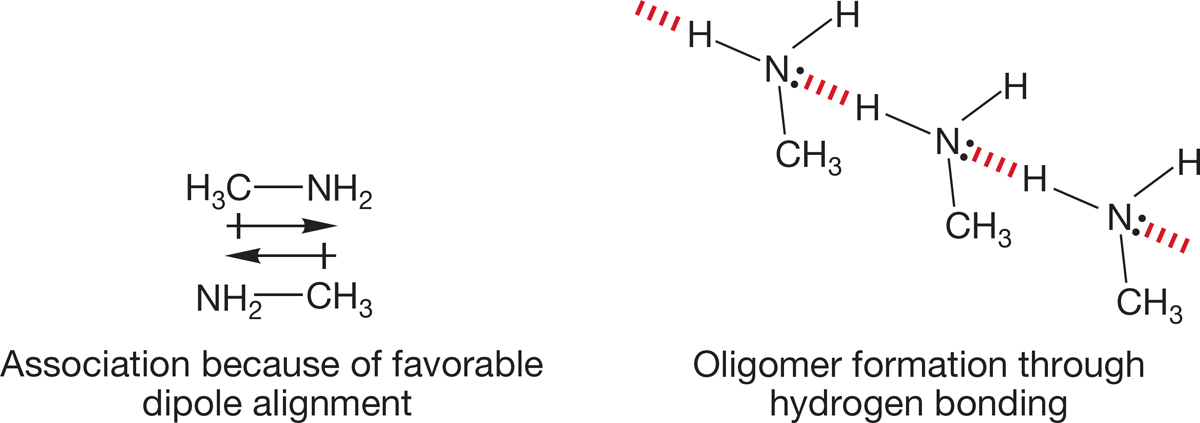
FIGURE 6.25 The effective molecular weight of amines is increased through association and hydrogen bonding.
Even though these intermolecular hydrogen bonds are relatively weak (~5 kcal/mol) they are critically important. For example, life as we know it is clearly not possible without water, and there would be no liquid water without hydrogen bonds. Moreover, hydrogen bonds are used to maintain the proper structures in proteins and nucleic acids, polymeric structures essential to our existence that we will meet later in this book.
Amines, like alcohols, have much higher boiling points than those of hydrocarbons of similar molecular weight, although the effect is not as great as with alcohols (Table 6.4; compare to Table 6.3). The reason for the increased boiling points is the same as for alcohols. Amines are polar compounds and aggregate in solution. Like alcohols, small amines are miscible with water. Boiling requires overcoming the intermolecular attractive forces between the dipoles. Moreover, amines form hydrogen-bonded oligomers in solution, effectively increasing the molecular weight and further increasing the boiling point (Fig. 6.25).
When hydrogen bonding is impossible, as with tertiary amines, boiling points decrease (Table 6.4).
TABLE 6.4 Some Physical Properties of Amines and a Few Related Alkanes
Compound |
bp (ºC) |
mp (ºC) |
Density (g/mL) |
NH3 |
–33.4 |
–77.7 |
0.77 |
CH3NH2 |
–6.3 |
–93.5 |
0.70 |
CH3CH3 |
–88.6 |
–183.3 |
0.57 |
CH3CH2NH2 |
16.6 |
–81 |
0.68 |
CH3CH2CH3 |
–43.1 |
–189.7 |
0.59 |
(CH3)2NH |
7.4 |
–93 |
0.68 |
(CH3)3N |
2.9 |
–117.2 |
0.64 |
|
184 |
–6.3 |
1.02 |
bp, boiling point; mp, melting point.
It is impossible to discuss the properties of amines without mentioning smell. The smell of amines ranges from the merely fishy to the truly vile. Indeed, the odor of decomposing fish owes its characteristic unpleasant nature to amines. Diamines are even worse. Some indication of how bad-smelling they often are can be gained from their names. 1,4-Butanediamine is called putrescine, and 1,5-pentanediamine is called cadaverine.
PROBLEM 6.11 Many people use lemon when eating fish. This custom is a carryover from the days when it was difficult to preserve fish, and the lemon acted to diminish the unpleasant odor (if not the decomposition). Lemons contain 5–8% citric acid, and this acidity contributes to their sour taste. Explain why lemon juice is effective at reducing the odor of fish.
Ethers are polar, but only for the smallest members of the class does this polarity strongly affect physical properties. Other ethers are sufficiently hydrocarbon-like so as to behave as their all-carbon relatives. For example, diethyl ether has nearly the same boiling point as pentane and is only modestly soluble in water. Table 6.5 gives some physical properties for a few common ethers.
6.4b Acidity and Basicity Alkyl halides are neither substantial acids nor bases. They are not proton donors and have no appreciably basic electron pairs. Alcohols and amines, however, can be proton donors and, because of their lone-pair electrons, can be bases as well.
We have already seen water and alcohols acting as Brønsted acids and bases. Hydrogen bonding is a perfect example of such behavior. In Chapter 7, we are going to look closely at several reactions of alcohols. These processes depend on the ability of alcohols to act as both acids and bases. Accordingly, let’s first make sure that we can see these roles clearly.
TABLE 6.5 Some Physical Properties of Ethers
Compound |
bp (ºC) |
mp (ºC) |
Density (g/mL) |
H3C―CH3―O |
–23 |
–138.5 |
0.668 |
CH3CH2―O―CH2CH3 |
34.6 |
–119.2 |
0.725 |
|
|
| |
H3C―O―CH2CH3 |
7.6 |
–139 |
0.714 |
|
|
| |
(CH3)3C―O―CH3 |
55.2 |
–109 |
0.740 |
|
|
| |
CH3CH2―O―CH |
35.5 |
–115.8 |
0.759 |
|
31.4 |
–85.6 |
0.951 |
|
67 |
–108 |
0.889 |
|
155.0 |
–37.5 |
0.996 |
|
|||
bp, boiling point; mp, melting point.
A Brønsted acid is any compound that can donate a proton. A Brønsted base is any compound that can accept a proton. Let’s look first at a very simple process, the reaction of solid potassium hydroxide (KOH) with gaseous hydrochloric acid (HCl). This reaction is nothing but a competition of two Brønsted bases (Cl− and HO−) for a proton, H+. The stronger Brønsted base (HO−) wins the competition (Fig. 6.26).

FIGURE 6.26 Two Brønsted bases (blue) competing for a proton.
Hydrochloric acid (HCl) is called the conjugate acid of Cl−, and Cl− is the conjugate base of hydrochloric acid. Conjugate acids and bases are related to each other through the gain and loss of a proton (Fig. 6.27).
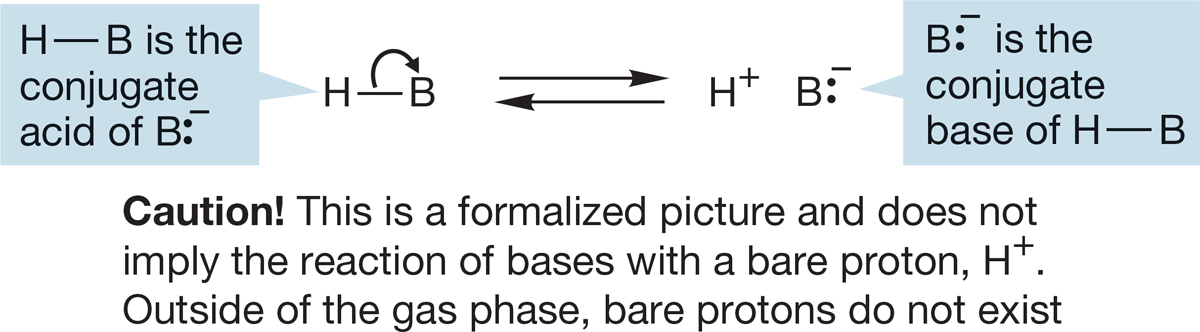
FIGURE 6.27 Conjugate acids and bases.
PROBLEM 6.12 What are the conjugate acids of the following molecules?
(a) H2O
(b) −OH
(c) NH3
(d) CH3OH
(e) H2C O
O
(f) −CH3
PROBLEM 6.13 What are the conjugate bases of the following molecules?
(a) H2O
(b) −OH
(c) NH3
(d) CH3OH
(e) CH4
(f) HOSO2OH
(g) −OSO2OH
As we saw in Figure 6.24, a molecule may be either a Brønsted acid or a Brønsted base. Consider water, for example. Water can both donate a proton (act as a Brønsted acid) and accept a proton (act as a Brønsted base). Figure 6.28 shows two such reactions: (a) the protonation of water by hydrogen chloride, in which water acts as a base, and (b) the protonation of hydroxide ion by water, in which water acts as the acid.
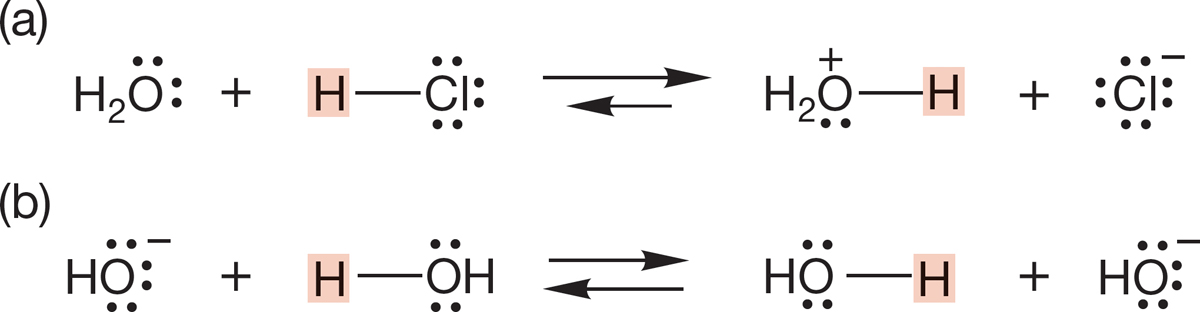
FIGURE 6.28 Water is both a Brønsted acid and base.
Many reactions begin with a protonation step. Because strong acids are better able to donate a proton than are weaker acids, it will be important for us to know which molecules are strong acids and which are weak acids. The dissociation of an acid HA in water can be described by the equation
HA + H2O ⇄ H3O+ + A−
This equation leads to an expression for the equilibrium constant, K.
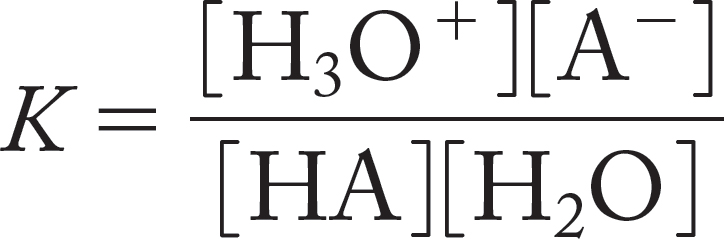
Because it is present as solvent, in vast excess, the concentration of water remains constant in the ionization reaction. This equation is usually rewritten to give the acidity constant, Ka,
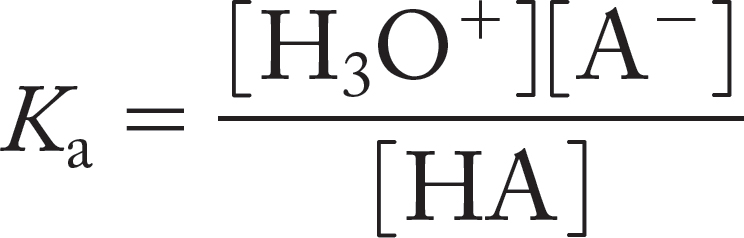
By analogy with pH, we can define a quantity pKa.
pKa = −log Ka
The stronger the acid, the lower is its pKa. Table 6.6 gives pKa values for some representative compounds. This short list spans about 80 powers of 10 (pKa is a log function). In practice, any compound with a pKa lower than about +5 is regarded as a reasonably strong acid; those with pKa values below 0 are very strong. As we discuss new kinds of molecules, more pKa values will appear. Should you memorize this list? We think not, but you will need to have a reasonable idea of the approximate acidity of different kinds of molecules.
TABLE 6.6 Some pKa Values for Assorted Moleculesa
Compound |
pKa |
Compound |
pKa |
HI |
−10 |
H4N+ |
9.2 |
HBr |
−9 |
CH3OH |
15.5 |
HCl |
−8 |
H2O |
15.7 |
H2SO4 |
−3 |
ROH |
16–18 |
|
−2 |
HC |
24 |
H3O+ |
−1.7 |
NH3 |
38 |
HNO3 |
−1.3 |
(CH2)3 (cyclopropane) |
46 |
HF |
3.2 |
H2C |
50 |
RCOOH |
4–5 |
CH4 |
50–60 |
H2S |
7.0 |
(CH3)3CH |
50–70 |
|
|||
a When there is a choice, it is the underlined H that is lost. A longer list is provided on the back inside cover of the book. | |||
In alcohols and water, Brønsted basicity is centered on the nonbonding electrons of the oxygen atom, which form bonds with a variety of acids. Protonation converts an alcohol, ROH, into an oxonium ion (RO+H2). This conversion has profound chemical consequences, which we can preview here. Breaking the R―OH bond to form ions is very difficult, because both positive (R+) and negative (HO−) ions must be formed. Hydroxide, HO−, is very difficult to form—we say that hydroxide is a poor leaving group—and alcohols do not ionize easily (Fig. 6.29). After protonation, however, the “leaving group” is no longer hydroxide, but water. Formation of the neutral molecule water is a much easier process (Fig. 6.29).
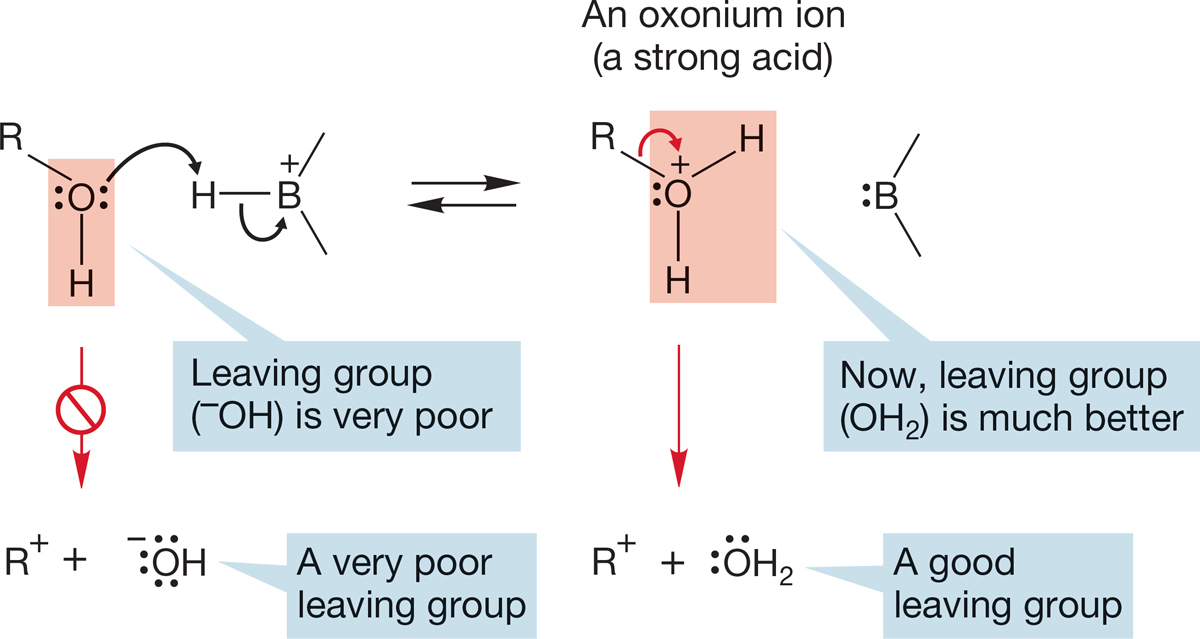
FIGURE 6.29 Protonation of an alcohol leads to an oxonium ion. In this process, the very poor leaving group OH is transformed into the good leaving group OH2.
Protonated alcohols are very strong Brønsted acids. Therefore, in order to protonate an alcohol, it takes a very strong acid indeed. The conjugate base (B:−) of the protonating acid (HB) must be a weaker base than the alcohol. The base must be an ineffective competitor in the equilibrium of Figure 6.29.
Oxonium ions are much better proton donors (acids) than are alcohols themselves. Table 6.7 gives the pKa values for a few protonated alcohols and water. Oxonium ions formed from alcohols have pKa values in the −2 range, and those from ethers (R―O―R′) are even more acidic.
TABLE 6.7 Some pKa Values for Simple Oxonium Ions
Compound |
pKa |
|
−1.74 |
|
−2.2 |
|
−1.9 |
|
−2.3 |
|
−2.2 |
|
−2.6 |
|
About −3.5 |
|
|
Brønsted acidity is centered on the hydroxyl hydrogen. Loss of this hydrogen to an acceptor, a Brønsted base, leads to the conjugate base of the alcohol, the alkoxide ion (RO−). The proton is not just lost as H+ but removed by a base (Fig. 6.30).
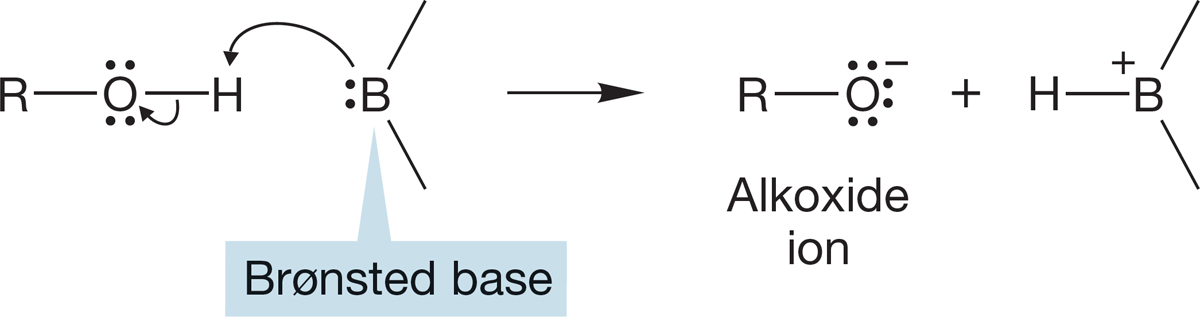
FIGURE 6.30 Loss of a proton to a general Brønsted base leads to an alkoxide ion. Alkoxides are named very simply as oxides of the parent alcohol. Thus, methyl alcohol, CH3OH, is deprotonated to give methoxide, CH3O−; ethyl alcohol, CH3CH2OH, is deprotonated to give ethoxide, CH3CH2O−; and so on.
Alcohols are approximately as acidic as water. Table 6.8 gives the pKa values for some simple alcohols in aqueous solution and for water itself.
TABLE 6.8 Some pKa Values for Simple Alcohols in Water
Compound |
|
pKa (Loss of Underlined H) |
Water |
H2O |
15.7 |
Methyl alcohol |
CH3OH |
15.5 Strongest acid |
Ethyl alcohol |
CH3CH2OH |
15.9 |
Isopropyl alcohol |
(CH3)2CHOH |
16.5 |
tert-Butyl alcohol |
(CH3)3COH |
17.0 Weakest acid |
|
||
Note that the acidity order in water is CH3OH > CH3CH2OH > (CH3)2CHOH > (CH3)3COH. Apparently, the more alkyl groups on the alcohol, the weaker an acid it is. For many years, this pKa order was explained by assuming that alkyl groups are intrinsically electron donating. If this were true, the product alkoxides would be destabilized by alkyl groups, and the acidity of the corresponding alcohol would be reduced (Fig. 6.31).
Inductive effects, which result from polarized sigma bonds, are important. For example, the pKa of 2,2,2-trifluoroethanol (CF3CH2OH) is 12.5, whereas the pKa of ethyl alcohol is 15.9. The fluorinated alcohol is more than a thousand times as acidic as ethyl alcohol (remember the pKa scale is logarithmic). Fluorinated alcohols are always stronger acids than their hydrogen-substituted counterparts.
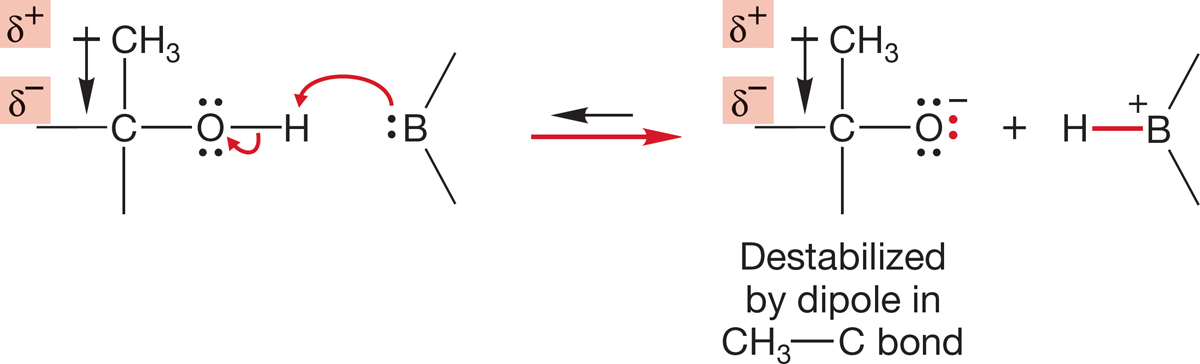
FIGURE 6.31 If alkyl groups were electron-releasing, formation of an alkoxide from an alcohol would be retarded by alkyl groups.
WORKED PROBLEM 6.14 Explain in detail why 2,2,2-trifluoroethanol is a stronger acid than ethyl alcohol.
ANSWER Fluorine is very electronegative. Therefore, the fluorines are strongly electron withdrawing inductively, and that effect helps to stabilize the product alkoxide ion. The fluorines also stabilize the transition state leading to the alkoxide because partial negative charge has begun to develop on the oxygen atom. So the fluorines have an effect on both the kinetic (the transition state of the deprotonation) and thermodynamic (the stability of the conjugate base after deprotonation) properties of the alcohol.
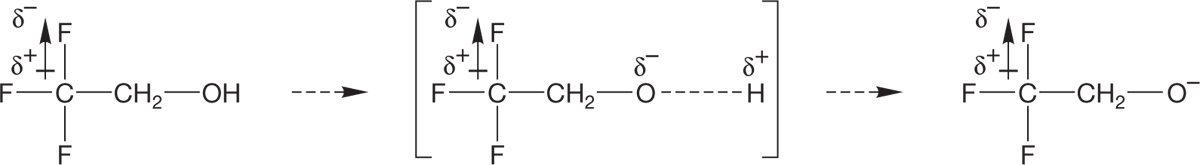
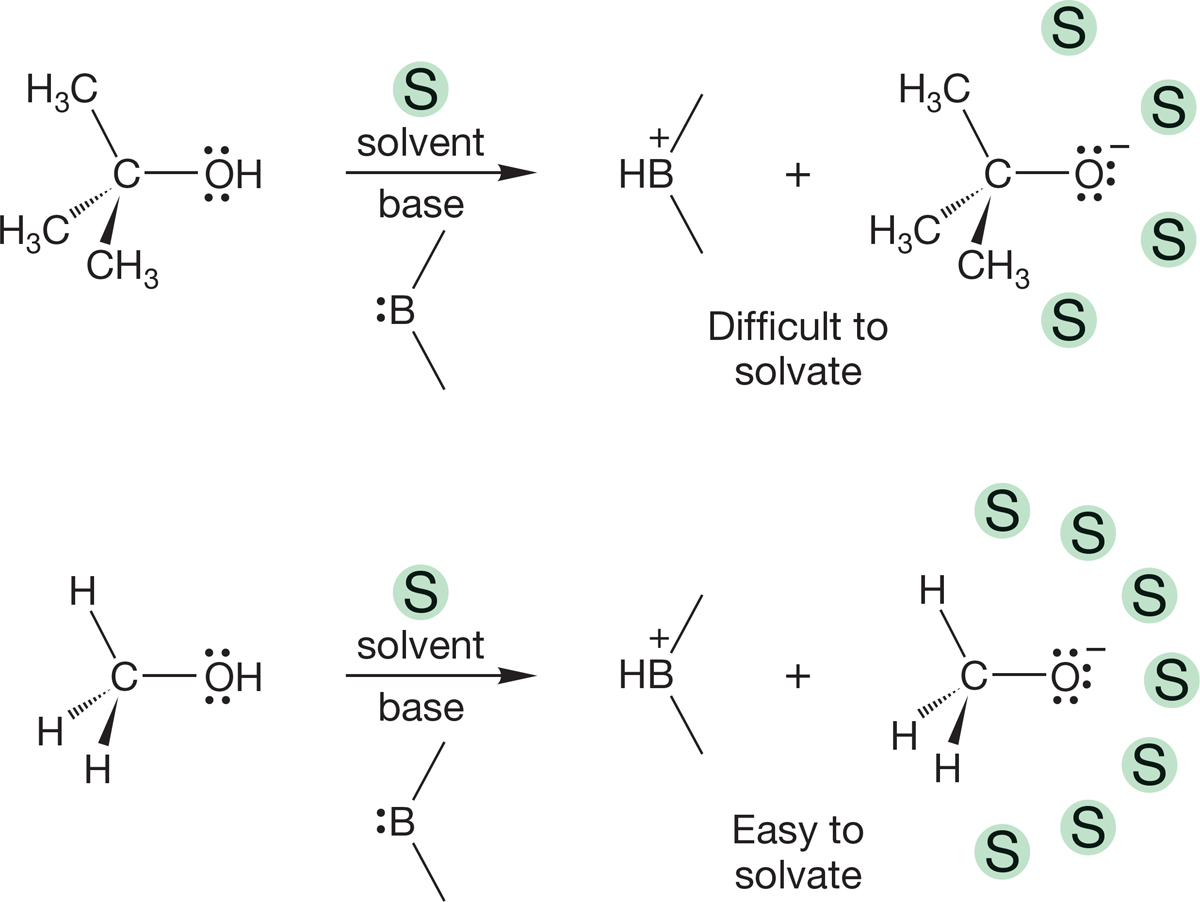
FIGURE 6.32 The smaller the alkoxide ion, the easier it is for solvent molecules to approach and stabilize it.
Yet this traditional, and simple, explanation that treats alkyl groups as intrinsically electron-donating is not correct. In 1970, Prof. John Brauman (b. 1937) and his co-workers at Stanford University showed that in the gas phase, the opposite acidity order is obtained. The intrinsic acidity of the four alcohols of Table 6.8 is exactly opposite to that found in solution. The acidity order measured in solution reflects a powerful effect of the solvent, not the natural acidities of the alcohols themselves. Organic ions are almost all unstable species, and the formation of the alkoxide anions depends critically on how easy it is to stabilize them through interaction with solvent molecules, a process called solvation. tert-Butyl alcohol is a weaker acid in solution than methyl alcohol because the large tert-butyl alkoxide ion is difficult to solvate. The more alkyl groups, the more difficult it is for the stabilizing solvent molecules to approach (Fig. 6.32). In the gas phase, where solvation is impossible, the natural acidity order is observed.
In the gas phase, the alkyl groups actually operate so as to stabilize the charged alkoxide ions by allowing electron delocalization into alkyl group empty antibonding orbitals, exactly the opposite from what had been thought (Fig. 6.33).
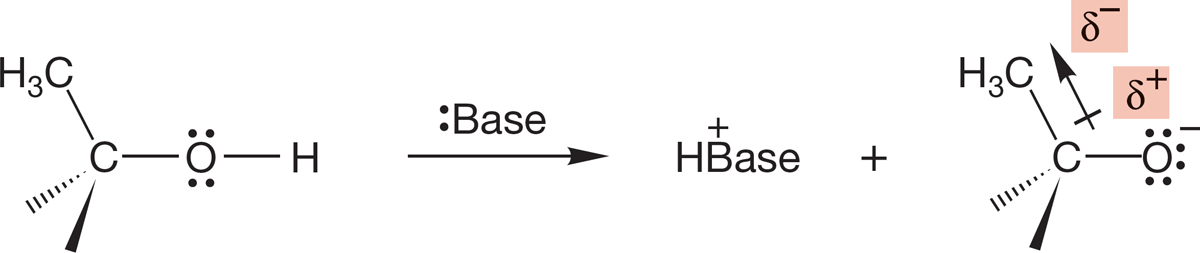
FIGURE 6.33 Alkyl groups can stabilize the alkoxide.
WORKED PROBLEM 6.15 Can you describe this stabilization of the conjugate base in orbital terms? No orbital construction or complicated argument is necessary. A simple statement is all that is needed.
ANSWER Alkyl groups have both filled and empty molecular orbitals (see the problems at the end of Chapter 1 for several examples). A pair of electrons adjacent to an alkyl group can be stabilized through overlap with the LUMO of the alkyl group. (Similarly, an alkyl group stabilizes an adjacent empty orbital through overlap with the alkyl HOMO.)
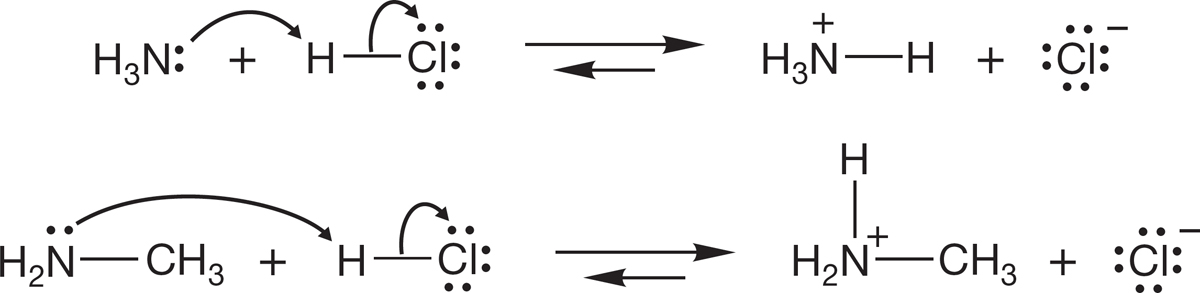
FIGURE 6.34 Ammonia and methylamine acting as bases.
What lessons are to be drawn from this discussion? First, solvation is important and not completely understood. We are certain to find other phenomena best explained in terms of solvation in the future. Second, the gas phase is the ideal medium for revealing the intrinsic properties of molecules. (Some would go further and say that calculation is the best way.) However, the practical world of solvated reactions is certainly real, and for us to understand reactivity we cannot afford to ignore the solvent. We will return to the subject of solvents and solvation in Section 6.5.
Like water and alcohols, amines are Brønsted bases (proton acceptors). Ammonia and primary, secondary, and tertiary amines are all weak bases (Fig. 6.34). Protonation of the nitrogen results in the formation of an ammonium ion. This process is significant in biological chemistry. When biological reactions need a base, it is often an amine that plays that role (Fig. 6.35).
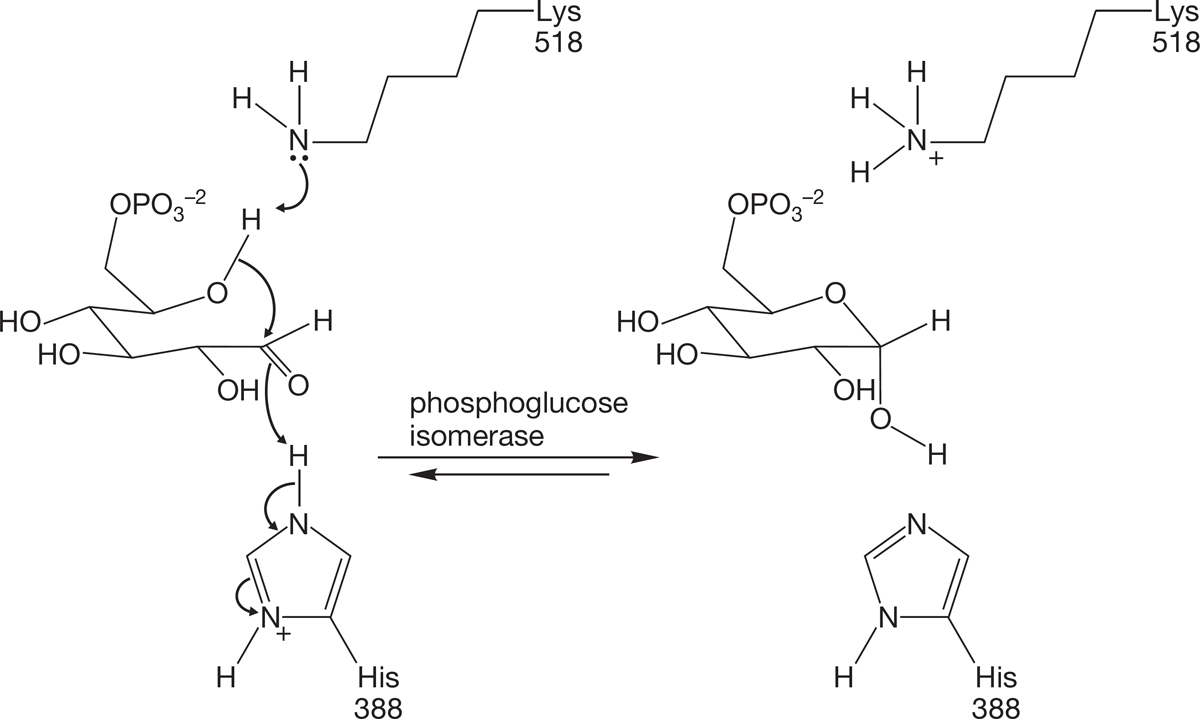
FIGURE 6.35 Amine groups are nature’s bases. Lys518 stands for a lysine amino acid at position 518 in a polyamino acid chain. His388 represents a histidine amino acid at position 388 of the same chain.
The better competitor an amine is in the proton-transfer reaction, the stronger is its basicity. A way of turning this statement around is to focus not on the basicity of the amine but on the acidity of its conjugate acid, the ammonium ion. If the ammonium ion is a strong acid, the related amine must be a weak base. If it is easy to remove a proton from the ammonium ion to give the amine, the amine itself must be a poor competitor in the proton-transfer reaction, which is exactly what we mean by a weak Brønsted base. Strongly basic amines give ammonium ions from which it is difficult to remove a proton: ammonium ions with high pKa values. So the pKa of the ammonium ion has come to be a common measure of the basicity of the related amine. Ammonium ions with high pKa values (weak acids) are related to strongly basic amines, and ammonium ions with low pKa values (strong acids) are related to weakly basic amines (Fig. 6.36).
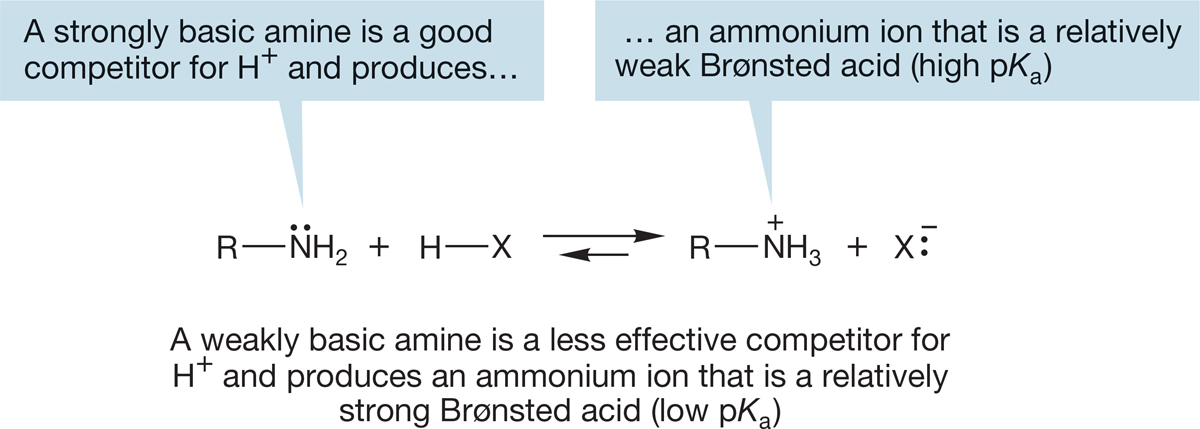
FIGURE 6.36 Ammonium ions with high pKa values are related to strongly basic amines, and ammonium ions with low pKa values are related to weakly basic amines.
Table 6.9 relates the basicity of ammonia and some very simple alkylamines by tabulating the pKa values of the related ammonium ions.
TABLE 6.9 Some pKa Values for Simple Ammonium Ions in Solution
Amine |
Formula |
Ammonium Ion |
pKa (in aqueous solution) |
Ammonia |
NH3 |
+NH4 |
9.24 |
Methylamine |
CH3NH2 |
|
10.63 |
Dimethylamine |
(CH3)2NH |
|
10.78 |
Trimethylamine |
(CH3)3N |
|
9.80 |
The general trend of the first three entries in Table 6.9 is understandable if we make the analogy between ammonium ions and carbocations. Remember (Chapter 3, p. 142): The more substituted a carbocation, the more stable it is. Similarly, the more substituted an ammonium ion, the more stable it is. The more stable the ammonium ion, the less readily it loses a proton and the higher its pKa. At least for the first three entries of Table 6.9, increasing substitution of the ammonium ion carries with it a decreasing ease of proton loss.
Actually, even the first three entries in Table 6.9 should make you suspicious. Why is there a much bigger change between ammonia and methylamine (1.4 pKa units) than between methylamine and dimethylamine (only 0.15 pKa units)? The structural change is the same in each case—the replacement of a hydrogen with a methyl group. When we get to the fourth entry, we find that things have truly gone awry: trimethylammonium ion is a stronger acid than the dimethylammonium ion and the methylammonium ion and is almost as strong as the ammonium ion itself, which implies that trimethylamine is a weaker base than dimethylamine or methylamine. There seems to be no continuous trend in these data.
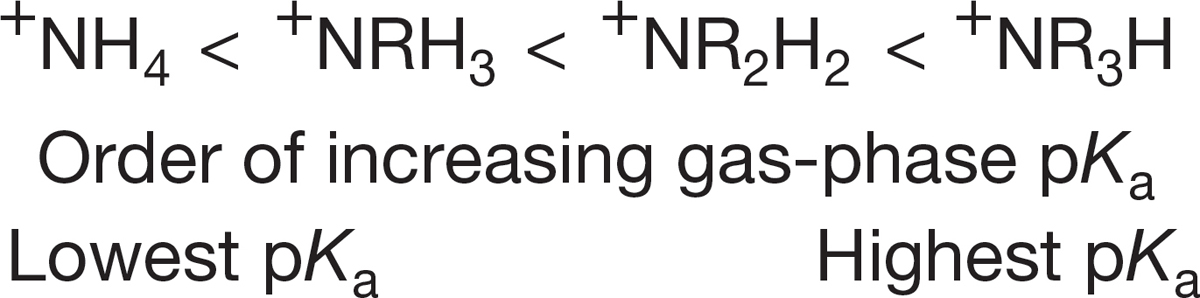
FIGURE 6.37 The gas-phase acidity of ammonium ions.
Indeed there isn’t, and it takes a look at matters in the gas phase to straighten things out. In the gas phase, the trend is regular. The basicity of amines increases with substitution, and the acidity of ammonium ions decreases with substitution (Fig. 6.37).
There must be some effect present in solution that disappears in the gas phase, and this effect must be responsible for the irregularities in Table 6.9. The culprit is the solvent itself. Ions in solution are strongly stabilized by solvation, by interaction of the solvent molecules with the ion. These interactions can take the form of electrostatic stabilization or of partial covalent bond formation, as in hydrogen bonding (Fig. 6.38).
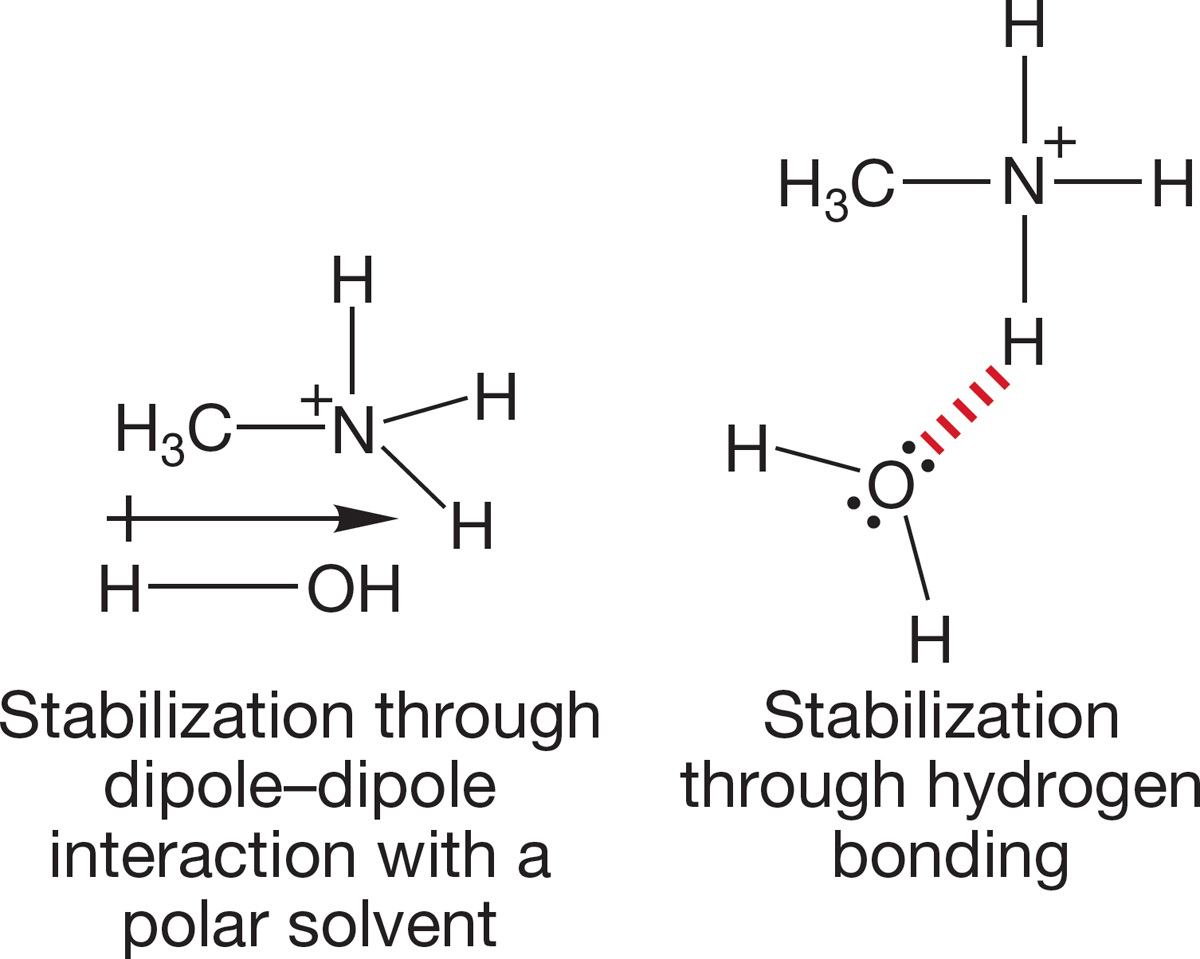
FIGURE 6.38 Stabilization of ammonium ions in solution.
In solution, an alkyl group has two effects on the stability of an ammonium ion. It stabilizes by helping the ammonium ion to disperse the charge, but it destabilizes the ion by interfering with solvation by making intermolecular solvation (charge dispersal) more difficult. These two effects operate in opposite directions. Little steric interference with solvation occurs when we replace one hydrogen of the ammonium ion with a methyl group, and the pKa change is more than a full pKa unit. This change reflects the stabilizing effect of the methyl group on the ammonium ion. However, when a second hydrogen is replaced by a methyl, the stabilization and interference with solvation nearly balance. The result is practically no change in the overall stability of the ammonium ion. When a third hydrogen is replaced with methyl, the destabilizing effects outweigh the stabilizing forces, and the result is a less stable, more acidic ammonium ion. In the gas phase, where there can be no solvation, only the stabilizing effects remain, and each replacement of a hydrogen with a methyl is stabilizing.
In addition, as we progress along the series +NH4, +NRH3, +NR2H2, +NR3H, +NR4, there are fewer hydrogens available for hydrogen bonding and therefore less stabilization of the ammonium ion in solution.
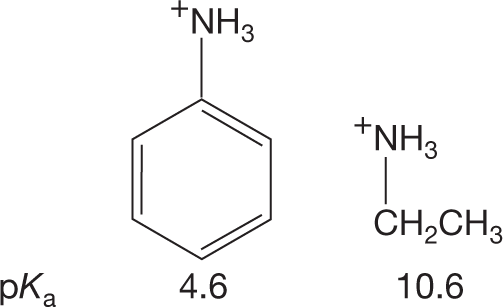
PROBLEM 6.16 Amines can be stabilized by other factors. Explain the following pKa data.
It must be admitted that this traditional discussion of base strength of amines in terms of the pKa of the related ammonium ion is indirect. Another, less common but undeniably more direct method is to frame the discussion in terms of the pKb (−log Kb), where Kb is the basicity constant of the amine (Fig. 6.39).
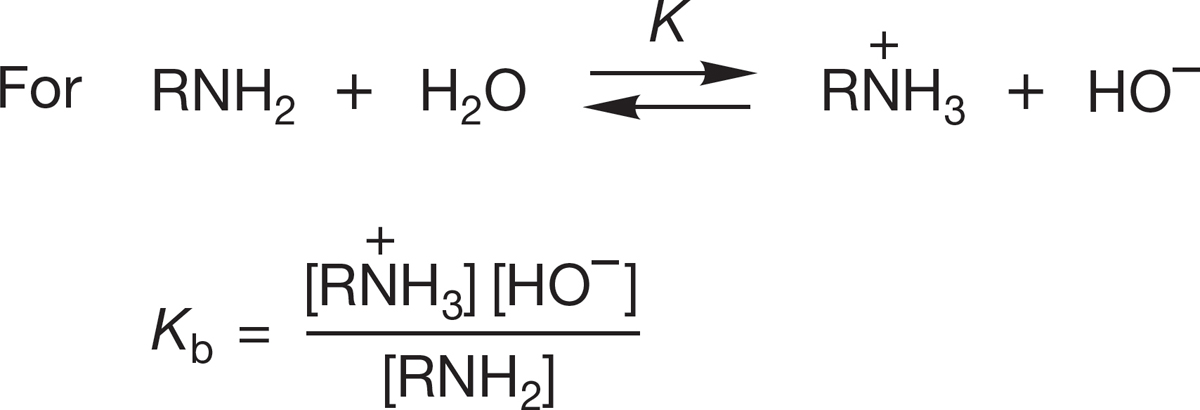
FIGURE 6.39 The expression for pKb.
The higher the pKb, the weaker is the base, just as the higher the pKa, the weaker is the acid. Typical amines have pKb values of about 4, which makes them quite strong bases compared to many other organic molecules.
Amines are much stronger bases than alcohols are. For example, it is much harder to deprotonate +NH4 than +OH3. The pKa value for the ammonium ion (9.2) is much higher than that for the oxonium ion (–1.7) (Fig. 6.40).
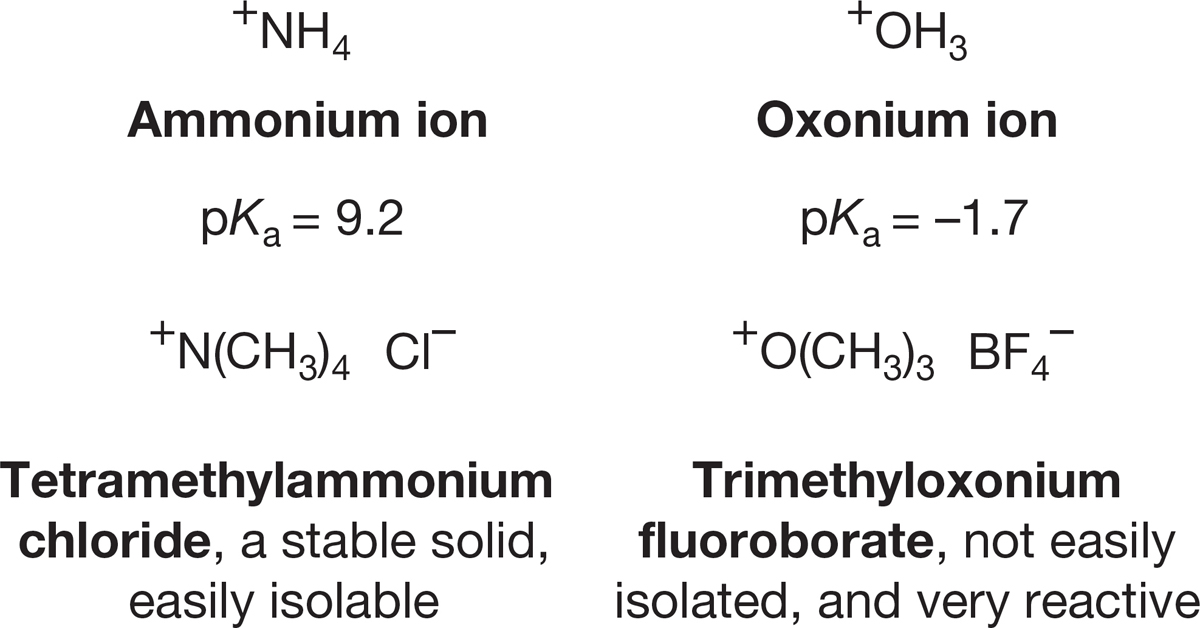
FIGURE 6.40 Ammonium ions are weaker acids than oxonium ions.
Why is the oxonium ion much more acidic than the ammonium ion? Oxygen is a more electronegative atom than nitrogen and bears the positive charge less well. We can see the results of the increased stability of the ammonium ion in practical terms. Stable ammonium salts are common, but salts of oxonium ions, though known, are rare and usually unstable (Fig. 6.40).
PROBLEM 6.17 The stability of oxonium ions depends on the nature of the negatively charged counterion. Tetrafluoroborate (−BF4) is an especially suitable counterion for an oxonium ion. This is because the tetrafluoroborate is not nucleophilic. Draw the Lewis structure of tetrafluoroborate. Why is it not a nucleophile?
Primary and secondary amines are Brønsted acids, but only very weak ones. Amines are much weaker acids than alcohols. Removal of a proton from an alcohol gives an alkoxide in which the negative charge is borne by oxygen. An amine forms an amide2 in which the less electronegative nitrogen carries the negative charge (Fig. 6.41).
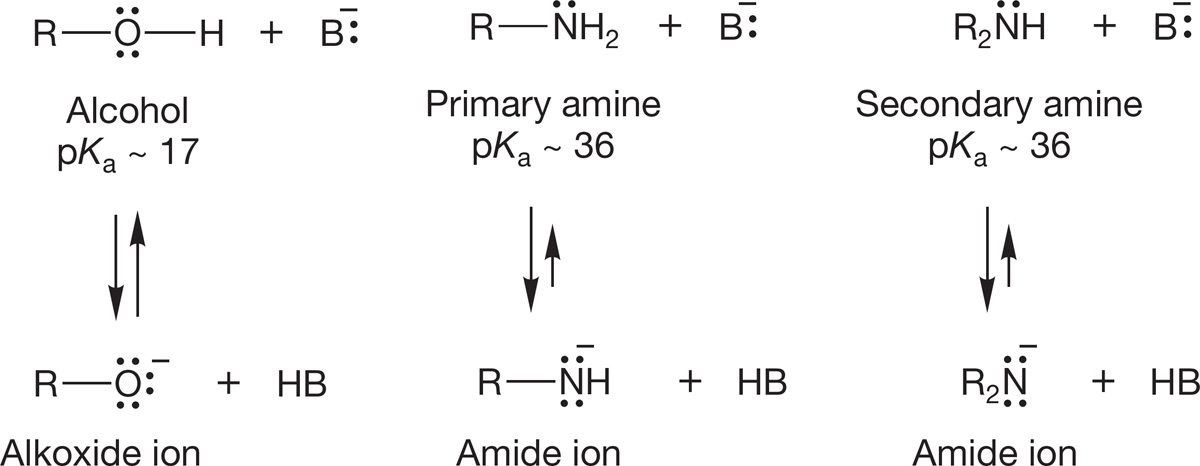
FIGURE 6.41 Primary and secondary amines are weak acids.
It takes a very strong base indeed to remove a proton from an amine to give the amide ion, which is itself a very strong base. Alkyllithium reagents (p. 257) are typically used (Fig. 6.42).
So, amines are good bases but relatively poor acids. Amide ions, the conjugate bases of amines, are even stronger bases than the amines themselves (Fig. 6.42).
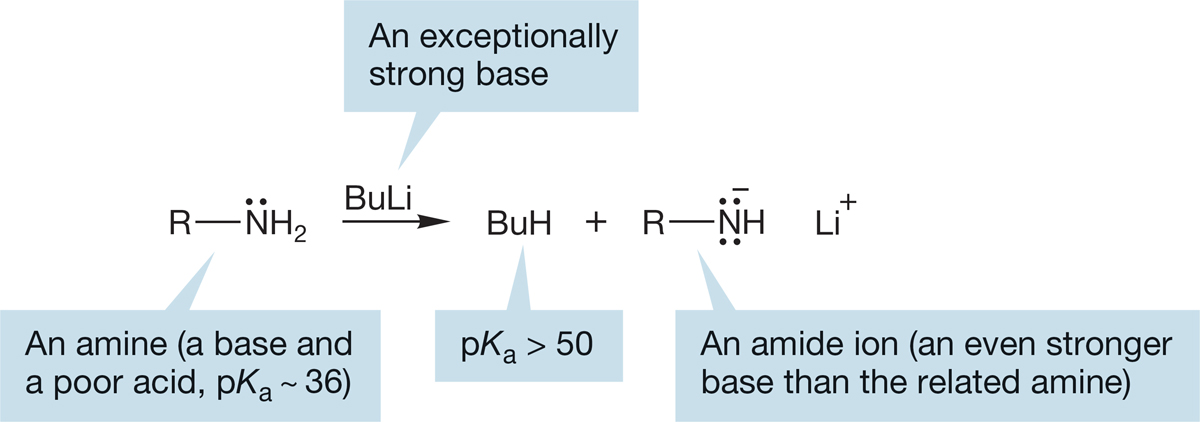
FIGURE 6.42 Very strong bases, such as alkyllithium reagents, can remove a proton from an amine to give an amide ion (Bu = CH2CH2CH2CH3).
PROBLEM SOLVING: ISHARE
Figuring out approximate or relative acidities of compounds is a very common problem and the key to many other problems. There are only two ways for you to deal with such problems. You can either memorize as many pKa values as you can and hope that nobody ever asks you for a different example or you can learn to work through the factors that influence acidity. The second path is far, far better than the first, because memorizing values does not prepare you for the (inevitable) moment when you need to know the acidity of a new compound.
There are many factors that influence acidity. We will work through the most important ones, using the acronym ISHARE, which stands for Induction, Size, Hybridization, Aromaticity, Resonance, and Electronegativity. Briefly, here is how to think about these factors.
Induction. Inductive effects are transmitted through sigma bonds. All bonds between two different atoms are polarized toward the more electronegative atom of the pair. The resulting dipole can stabilize (or destabilize) a nearby charge. For example, trichloroethanol has a pKa of 12.2, whereas ethanol itself has a pKa of 15.9. Thus, the halogens increase the acidity by nearly 4 pKa units. Why? Look at the conjugate bases formed by deprotonation of the two alcohols. The dipole in the C―Cl bond stabilizes the nearby negative charge by withdrawing electrons through the sigma bond framework (Fig. 6.43). That effect is called induction. Anything that stabilizes the conjugate base will make it easier to remove the hydrogen to get to that base (see Problem 6.14).
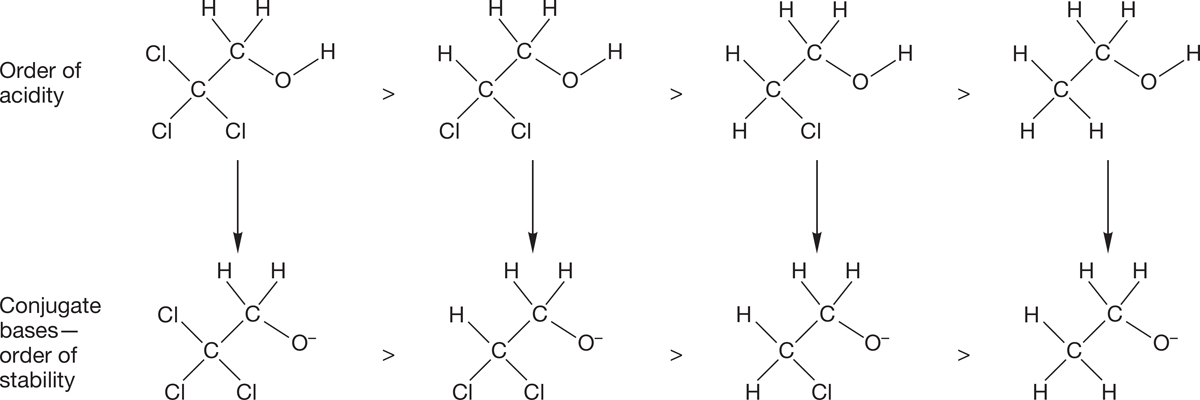
FIGURE 6.43 It is induction that stabilizes the conjugate base of a halogenated alcohol, which leads to its increased acidity.
Size. Generally, the larger an atom, the better it bears a negative charge. The larger nucleus, with more positively charged protons, stabilizes a negative charge better than does a smaller nucleus. In addition, the small hydrogen atom makes a strong bond to a similarly small atom such as fluorine and a very weak bond to larger atoms such as iodine because of poor orbital overlap. Thus, HF (pKa = 3.2) is a weaker acid than HI (pKa = −10) by a huge amount (Fig. 6.44). This is a comparison within the same column of the periodic table.
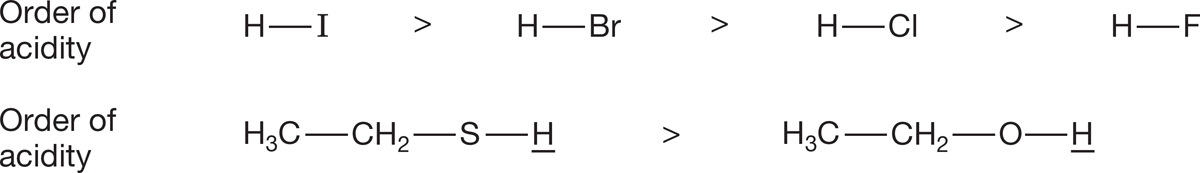
FIGURE 6.44 The size of the atom bonded to the hydrogen affects its acidity.
Hybridization. The more s character in an orbital, the lower in energy an electron in it is. Thus, an electron in an sp orbital is lower in energy than an electron in an sp2 orbital, which, in turn, is lower in energy than an electron in an sp3 orbital. As we saw in Chapter 3, this idea is exactly right: acetylene and terminal alkynes are far stronger acids than alkenes, and alkenes are far stronger acids than alkanes (Fig. 6.45). The conjugate base of acetylene is acetylide, and the acetylide anion is stabilized because electrons of the anion are in an sp-hybridized orbital.
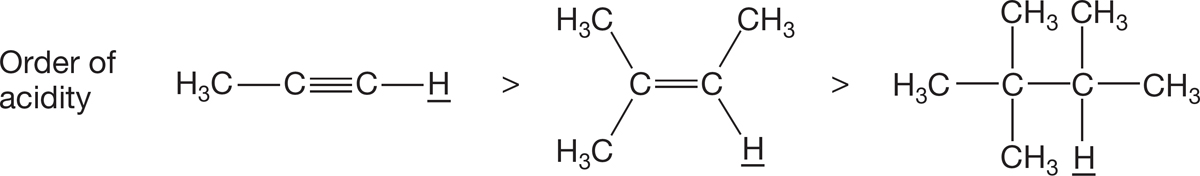
FIGURE 6.45 The hybridization of the atom bonded to the hydrogen affects its acidity.
Aromaticity. It turns out that we are not ready to discuss aromaticity yet. Stay tuned for Chapter 14. Although aromaticity has the most significant effect on acidity of all the factors, there are very few examples. Aromaticity is a factor when a molecule, as a result of deprotonation, goes from being not aromatic to being aromatic.
Resonance. Nonsolvated charged atoms are usually higher in energy than neutral atoms, which means that they are generally difficult to generate. It is especially difficult to form ions in which the charge is localized on one atom. As a result, a molecule that gives a delocalized anion upon deprotonation is a stronger acid than one that gives a localized anion. For example, carboxylic acids have pKa values around 4.5, whereas alcohols have pKa values in the range 15–20 (Fig. 6.46).
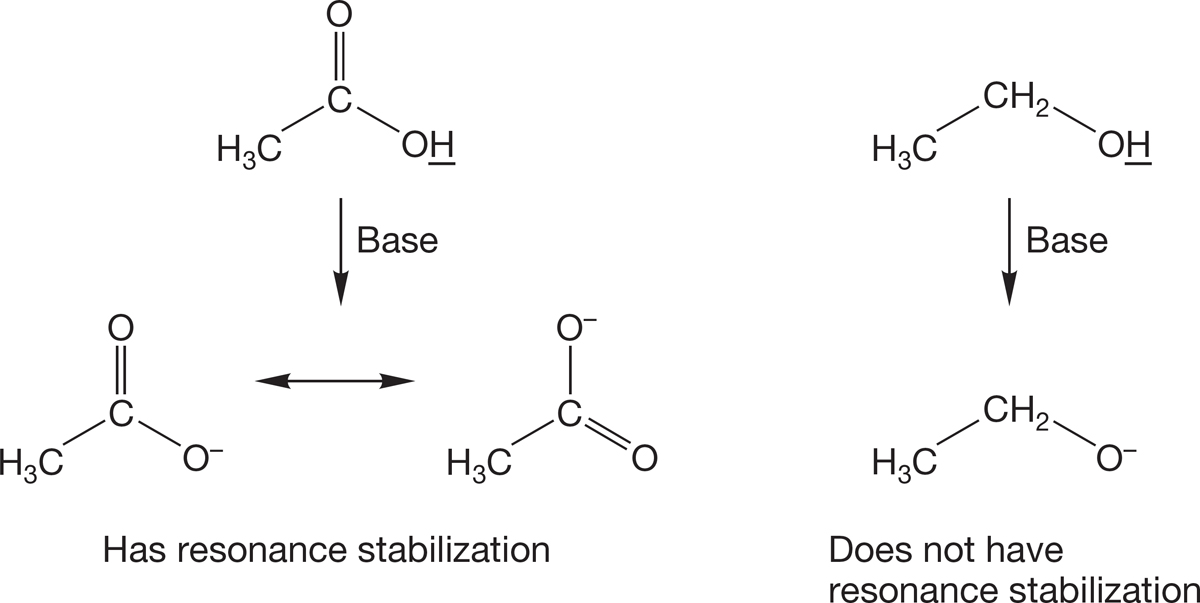
FIGURE 6.46 Resonance in the conjugate base affects the acidity.
Electronegativity. All other things being equal, forming an anion with the charge on a more electronegative atom is easier than putting that charge on a less electronegative atom. Thus, HF (pKa = 3.2) is a much stronger acid than an alcohol, ROH (pKa ~ 15.7; Fig. 6.47). This is a comparison within the same row of the periodic table.
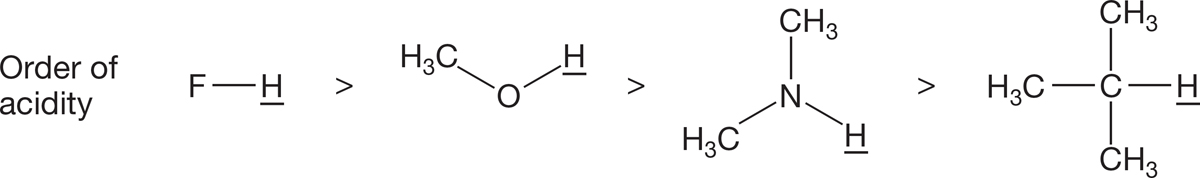
FIGURE 6.47 The electronegativity of the atom to which the hydrogen is attached affects the acidity of that hydrogen.
2Be careful—there is another kind of amide that has the structure R―CO―NH2. There is no written or verbal distinction made, so you need to know the context before you know which kind of amide is meant. We will use “amide ion” when referring to −NR2 to help avoid confusion.

 CH
CH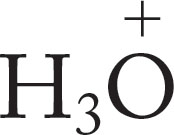
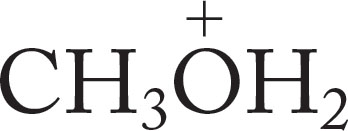



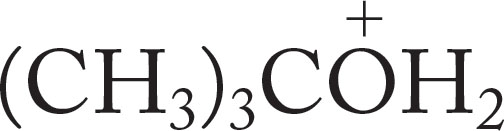

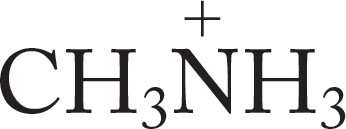
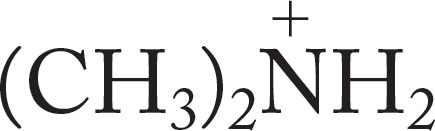
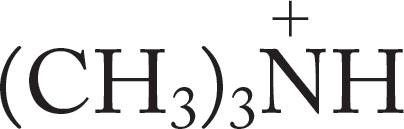
 CH3 ―F
CH3 ―F