6.6 Formation of Substituted Alkanes
In Chapter 3, we saw that alkyl halides can be made from alkenes (Fig. 6.52). In the next section, we will see one of the reasons why formation of alkyl halides is useful. We also learned that alcohols can be made from alkenes in Chapter 3. Formation of alcohols is particularly relevant to biological chemistry. Nature needs to use the alcohol functional group because it produces hydrogen bonding and polarity in the otherwise nonpolar hydrocarbons. These properties make the organic material soluble in water, which is nature’s solvent.
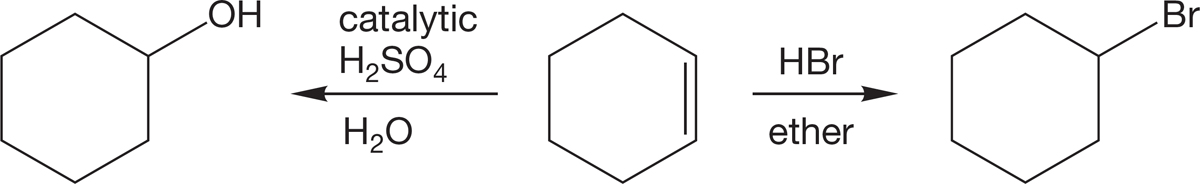
FIGURE 6.52 An example of the synthesis of alkyl halides or alcohols from an alkene.
There are many other ways to make alkyl halides and alcohols, which we will learn in subsequent chapters. We will also learn how to make ethers and amines. Alkyl halides and alcohols are the central functional groups (Fig. 6.53) for synthetic organic chemists. Alkyl halides are the only functional group that we can make directly from an alkane in the laboratory with any reasonable amount of control, and alkyl halides can be used to make all the functional groups discussed in this chapter.
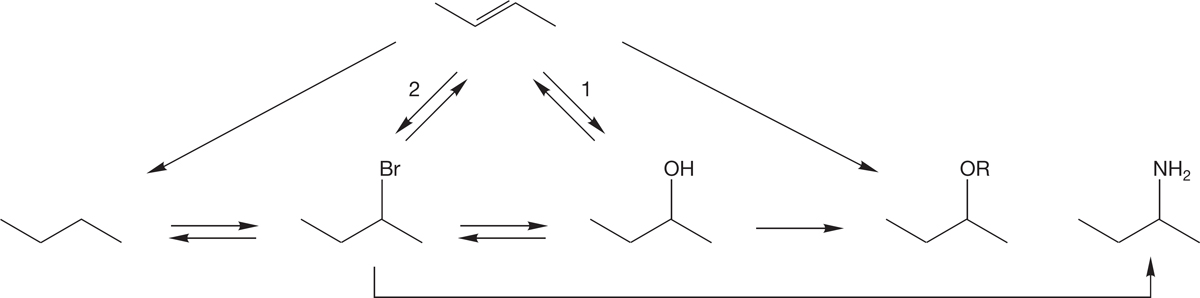
FIGURE 6.53 A reactivity scheme showing the routes for formation of alkyl halides, alcohols, ethers, and amines. We have already encountered the reactions labeled 1 and 2. Alcohols and alkyl halides can be made from alkenes. We will learn each of the remaining reactions in Chapters 7, 8, 10, and 12.