2.10 The Naming Conventions for Alkanes
Clearly, things are rapidly getting out of hand. Although it is not too difficult to remember the four types of butyl compounds (Fig. 2.35), remembering the eight types of pentyl compounds (Fig. 2.37) constitutes a tougher task—and the problem rapidly gets worse as the number of carbons increases. A system is absolutely necessary. In practice, the old common, nonsystematic names are retained through the butanes and for a few other often-used molecules. Once we reach five carbons, a systematic naming protocol developed by the International Union of Pure and Applied Chemistry (IUPAC) takes over. The IUPAC system is designed to handle any organic structure. There are IUPAC names for all the common names we have learned.
In general, the stem of the name tells you the number of carbon atoms (“meth” = 1, “eth” = 2, “prop” = 3, “but” = 4, and so on), and the suffix “ane” tells you that the molecule is an alkane. The alkane name is sometimes called the root word. The names of some straight-chain alkanes are collected in Table 2.4.
TABLE 2.4 Some Straight-Chain Alkanes
Name |
Formula |
mp (°C) |
bp (°C) |
|
Methane |
CH4 |
−182.5 |
−164 |
|
Ethane |
CH3CH3 |
−183.3 |
−88.6 |
|
Propane |
CH3CH2CH3 |
−189.7 |
−43.1 |
|
Butane |
CH3(CH2)2CH3 |
−138.4 |
−0.5 |
|
Pentane |
CH3(CH2)3CH3 |
−129.7 |
36.1 |
|
Hexane |
CH3(CH2)4CH3 |
−95 |
69 |
|
Heptane |
CH3(CH2)5CH3 |
−90.6 |
98.4 |
|
Octane |
CH3(CH2)6CH3 |
−56.8 |
125.7 |
|
Nonane |
CH3(CH2)7CH3 |
−51 |
150.8 |
|
Decane |
CH3(CH2)8CH3 |
−29.7 |
174.1 |
|
Undecane |
CH3(CH2)9CH3 |
−25.6 |
195.9 |
|
Dodecane |
CH3(CH2)10CH3 |
−9.6 |
216.3 |
|
Eicosane |
CH3(CH2)18CH3 |
36.8 |
343.0 |
|
Triacontane |
CH3(CH2)28CH3 |
66 |
449.7 |
|
Pentacontane |
CH3(CH2)48CH3 |
92 |
|
|
mp, melting point; bp, boiling point.
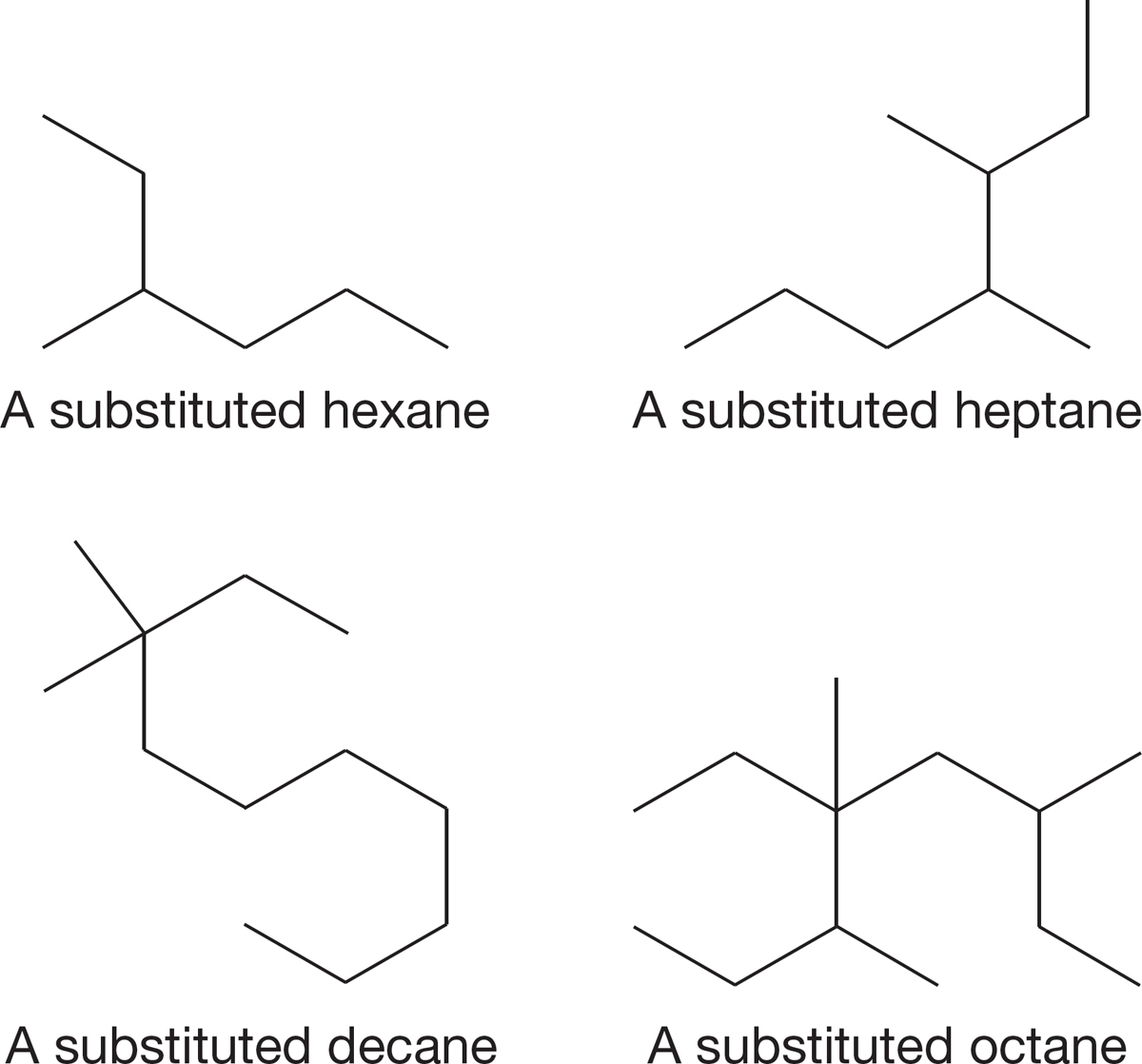
FIGURE 2.40 Finding the longest straight chain of carbons in alkanes.
It will be important to know how to interpret the IUPAC names, because this skill is part of learning the language of organic chemistry. This ability also will help you understand the contents listed on your cereal boxes and shampoo bottles. Here are the most important rules of the IUPAC naming convention for alkanes and substituted alkanes:
1. Find the longest chain of continuously connected carbons. Choose the root word that matches that chain length (see Table 2.4). The compound is named as a derivative of this parent hydrocarbon. Finding the longest chain can be a bit tricky at first because the angled, two- dimensional line drawings are not always drawn in a convenient horizontal line. The horizontal part of the drawing may not be the longest chain. In the examples in Figure 2.40, it is perfectly reasonable to count in any possible way in order to find the longest chain.
2. In a substituted alkane, the substituent is given a number based on its position in the parent hydrocarbon. The longest chain is numbered so as to make the number of the substituent position as low as possible. The substituent is listed as a prefix to the root word, with the number indicating its location on the chain. Some examples are shown in Figure 2.41. In a polysubstituted alkane, all the substituents receive numbers. The longest chain is numbered so that the lowest possible numbers are used. A useful shortcut is to number the chain from the end closest to the first substituent or branch point.
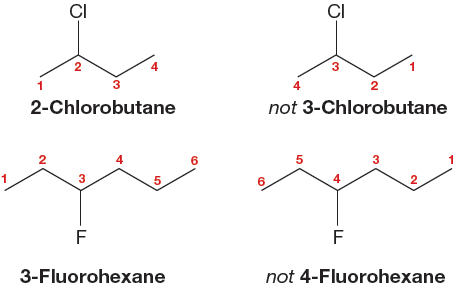
FIGURE 2.41 Number the chain so as to produce the smallest number for a substituent.
3. When there are multiple substituents, they are always ordered alphabetically in the prefix (Fig. 2.42). If the multiple substituents are identical, then di-, tri-, tetra-, and so on, are used as prefixes to the substituent name (Fig. 2.43). The modifiers di-, tri-, tetra-, and so on, are ignored in determining alphabetical order. Use of the common names for substituents is not strictly proper, but you might encounter it. In such cases, the sec- and tert- are ignored in determining alphabetical order, but the iso- and neo- are used. Thus, tert-butyl would appear in the name earlier than chloro, and isopropyl would come before methyl.
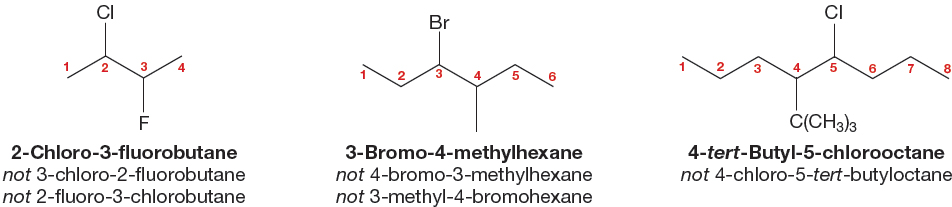
FIGURE 2.42 Nonidentical substituents are incorporated into the name alphabetically.
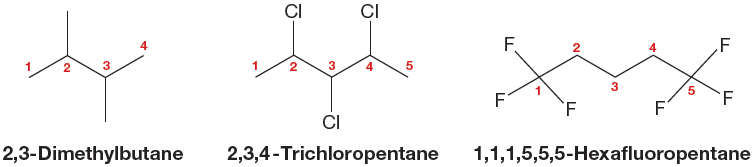
FIGURE 2.43 When the substituents in a polysubstituted alkane are the same, the prefixes di-, tri-, and so on, tell you the number of the multiple substituents.
4. When rules 1 through 3 do not resolve the issue, the name that starts with the lower number is used, as demonstrated in Figure 2.44. This rule is sometimes referred to as the alphabetical preference rule. If both numbering choices on the alkane use the same numbers, then the preference is given to the alphabet.
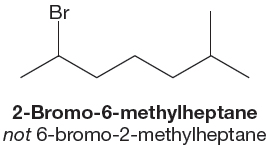
FIGURE 2.44 In unresolvable cases, start with the lowest possible number. The carbon chain is numbered from the end that gives the first-named substituent the lower number.
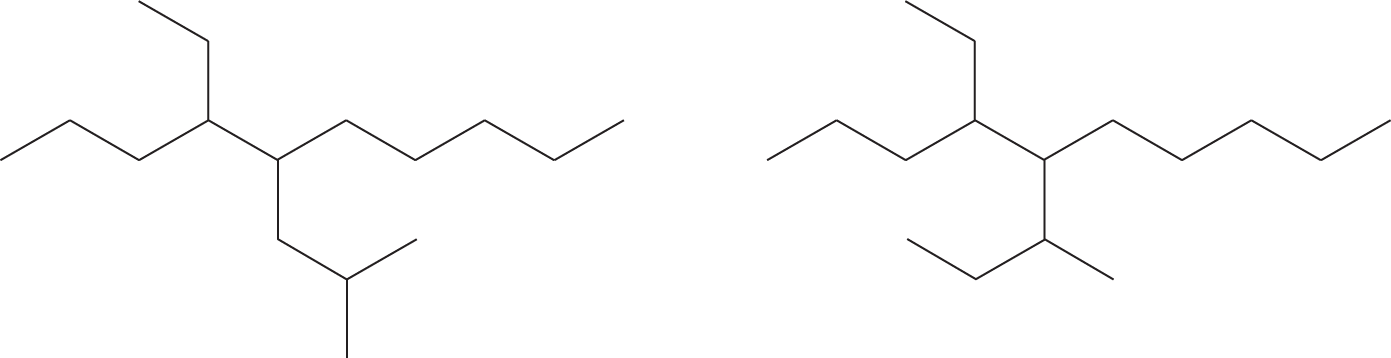
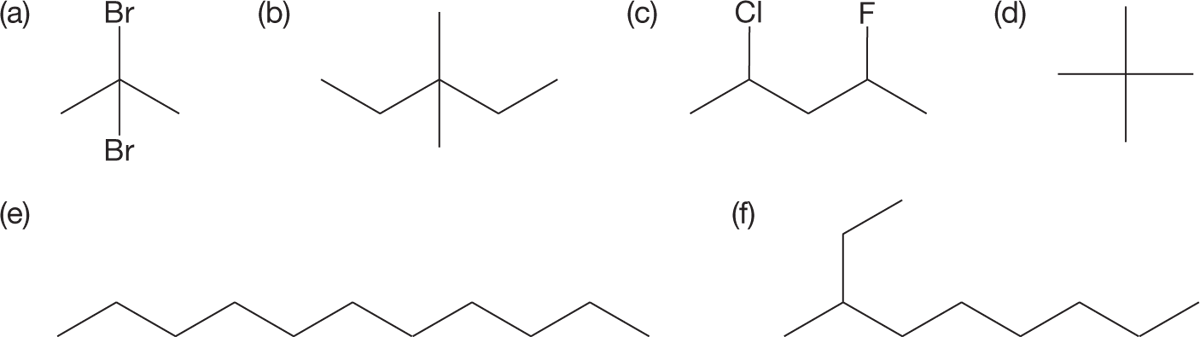
PROBLEM 2.25 Write systematic names for the following compounds:
PROBLEM 2.26 Write structures for the following compounds: (a) 2-bromobutane, (b) decane, (c) tert-butyl chloride, (d) 1,4-difluoropentane, (e) 2,4,4-trimethylheptane.
Although the instruction to name substituents on alkanes in alphabetical order looks simple, the process is a bit complex because some modifiers count in the alphabet and others do not. Table 2.5 contains a brief summary.
TABLE 2.5 Some Common Prefixes
Prefix |
Use |
Counts in Alphabetical Order? |
di- |
Any two identical groups |
No |
tri- |
Any three identical groups |
No |
tetra- |
Any four identical groups |
No |
iso- |
Isopropyl = (CH3)2CH― |
Yes |
neo- |
Neopentyl = (CH3)3CCH2― |
Yes |
sec- |
sec-Butyl = CH3CH2CH(CH3)― |
No |
tert- |
tert-Butyl = (CH3)3C― |
No |
PROBLEM 2.27 Use the information in Table 2.5 to name the following two compounds: