2.9 Pentanes (C5H12) and Pentyl Compounds (C5H11X)
We might now anticipate that four pentane compounds would emerge when we transform our four butyl―X compounds into five-carbon compounds by letting X = CH3 (Fig. 2.36). Yet only three different pentanes exist! Somehow the technique of generating larger alkanes by letting a general X become a methyl group has failed us. The problem lies again in the difficulty of representing three-dimensional structures on a two-dimensional surface. Two of the four structures in Figure 2.36 represent the same compound, even though in the two-dimensional representation they look different. The common names for the branched pentanes are isopentane and neopentane. We will not normally use these common names; in Section 2.10, we will describe a more systematic procedure for naming alkanes, including these two.
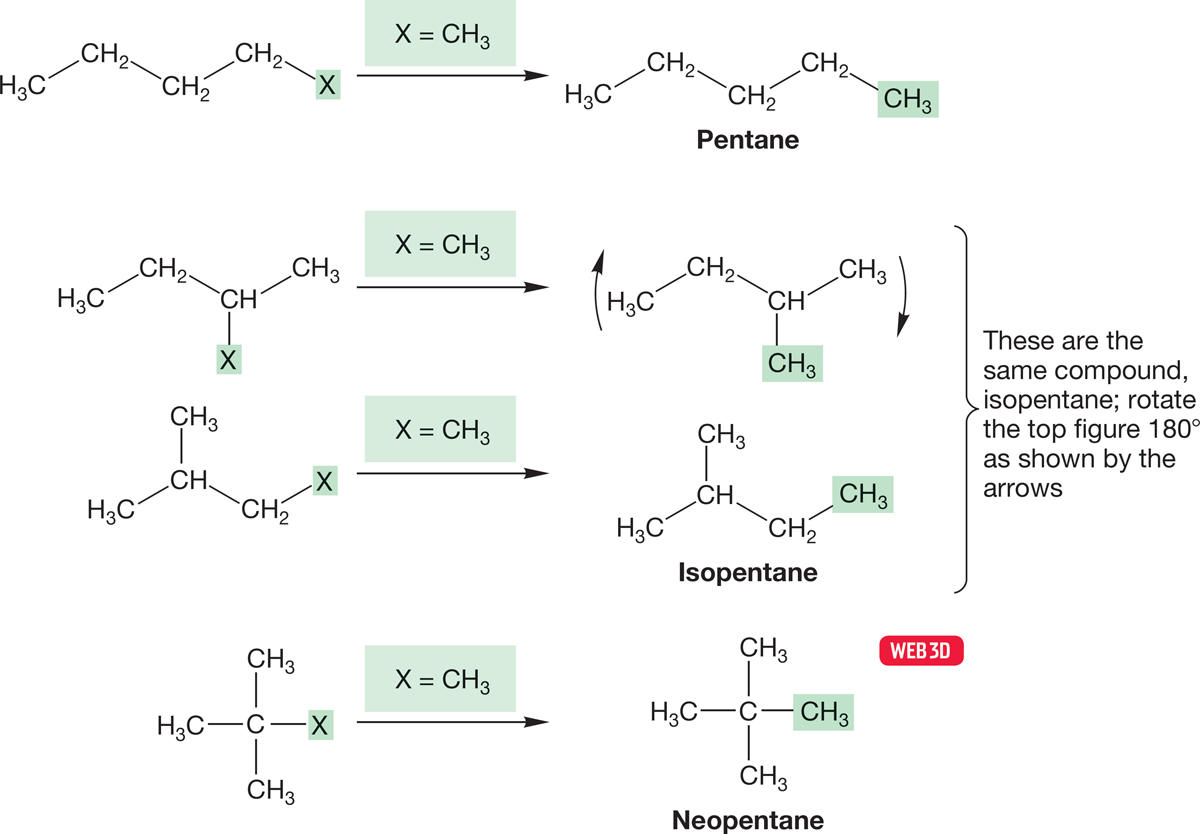
FIGURE 2.36 If we let X = CH3, it appears as though four C5H12 compounds should exist. Yet there are only three C5H12 compounds.
Even though there are only three pentane compounds—pentane, isopentane, and neopentane—Figure 2.37 shows that replacing one H in a pentane leads to no fewer than eight pentyl derivatives (C5H11—X). In the next section, we’ll see how to name these derivatives.
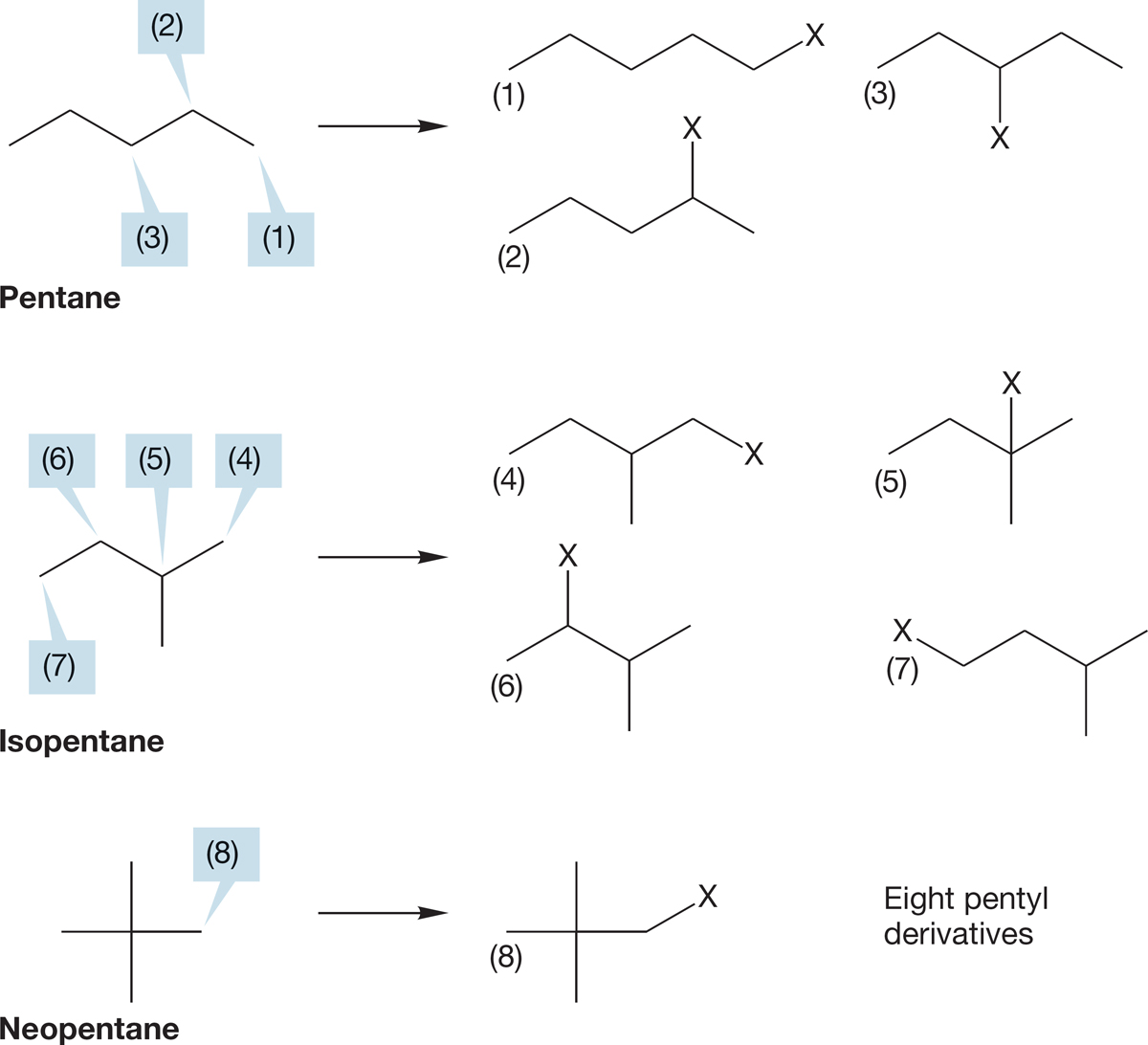
FIGURE 2.37 The eight pentyl derivatives formed by replacing hydrogens at the three different positions in pentane, the four different positions in isopentane, and the lone position in neopentane.
One important lesson to be learned from Figure 2.36 is that if you let it, the two-dimensional page will lie to you more often than not. As we have already seen, there is a tension between our need to communicate quickly and efficiently to one another and the accurate representation of these complicated organic structures. This tension is at least partly resolved by using codes, but the price of this use is that you must always be able to see past the code to the real, three-dimensional structure if you expect to be able to think effectively about organic chemistry. Because these codes become quite abstract, it is worth going through the process of developing the abstraction one more time.
Consider the representations for isopentane in Figure 2.38. The first drawing (Fig. 2.38a) only vaguely represents the real molecule. We then go to the more schematic structure of Figure 2.38b, and then to a structure in which the hydrogens are left out (Fig. 2.38c). Finally, we remove even the carbon labels, as in Figure 2.38d, to give the ultimate abbreviation for the structure of isopentane. Here nothing survives from the original picture except a representation of the backbone. You must be able to translate these highly schematic structures into three dimensions and to see the way these skeletal pictures transform into molecules.
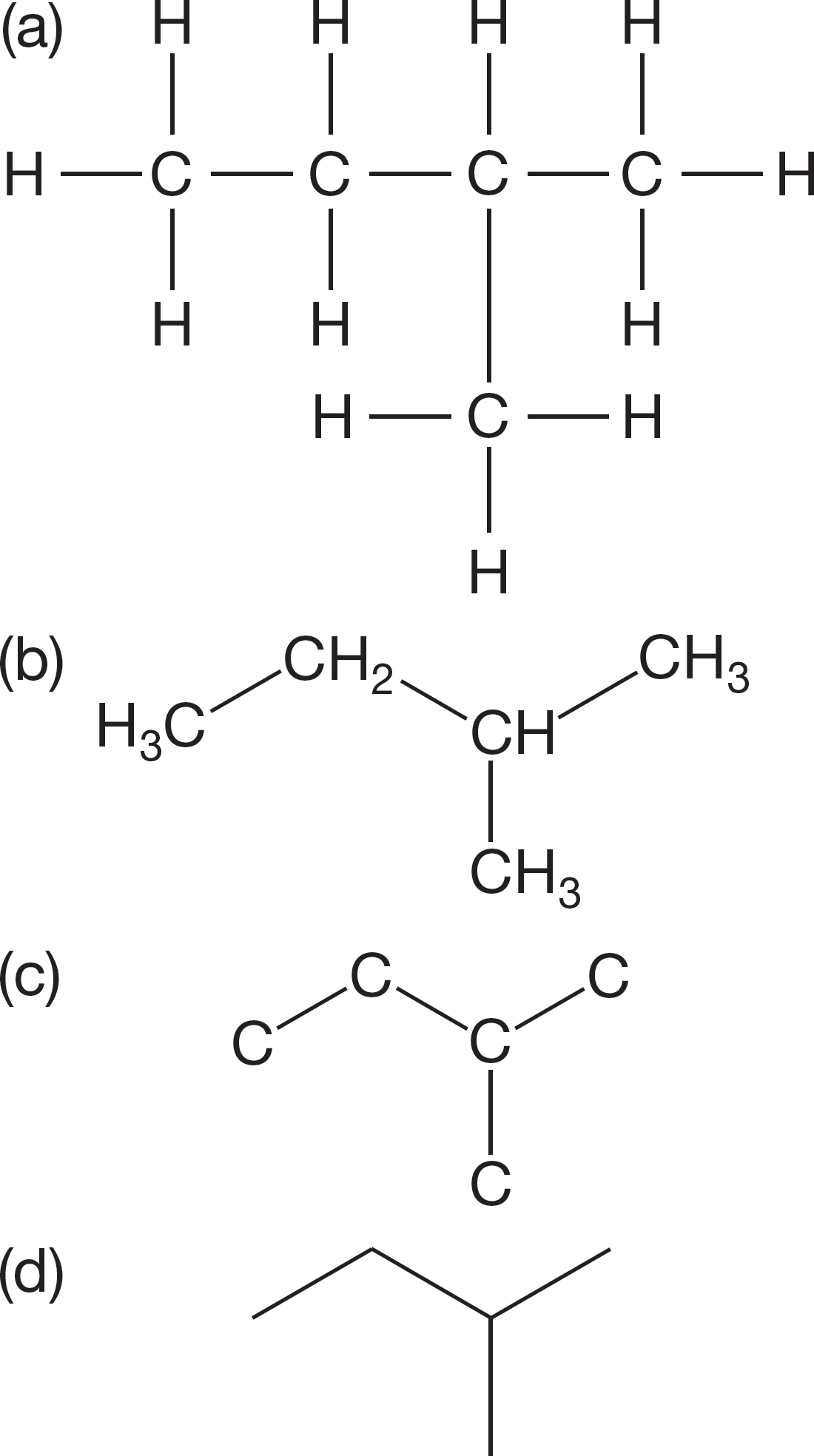
FIGURE 2.38 Four increasingly abstract representations of isopentane.
PROBLEM 2.24 Draw all the isomers of pentane, first in the format used in Figure 2.38a and then in the format used in Figure 2.38b.
Actually, even more can be done in our escape from the two dimensions of a book page. Line drawings can be approximated with models in which balls and sticks are used to represent atoms and the bonds between atoms. Other models concentrate on the bonds and leave it to you to imagine the atoms. Regardless of what kinds of models your class uses, they probably do a relatively poor job of showing the steric requirements of the real molecules. When we refer to steric requirements, we mean the volumes of space that atoms occupy. Chemists have developed space-filling models, which attempt to show the volumes of space carved out by the atoms. Figure 2.39 shows some representations of isopentane.
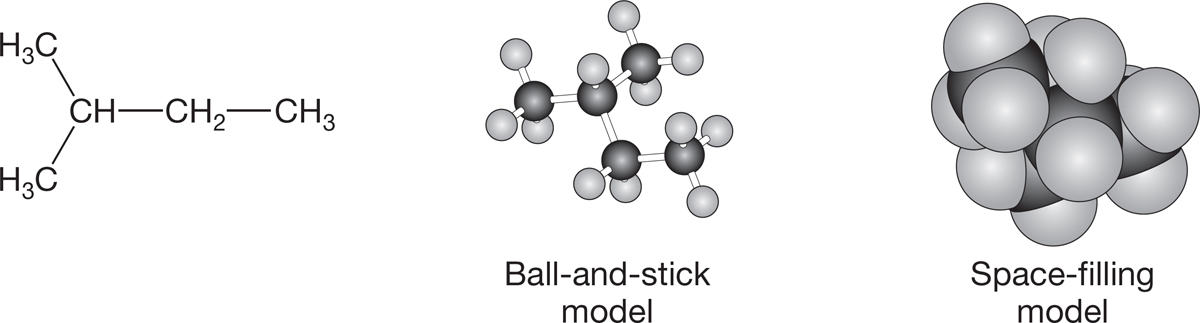
FIGURE 2.39 Views of isopentane.
Summary
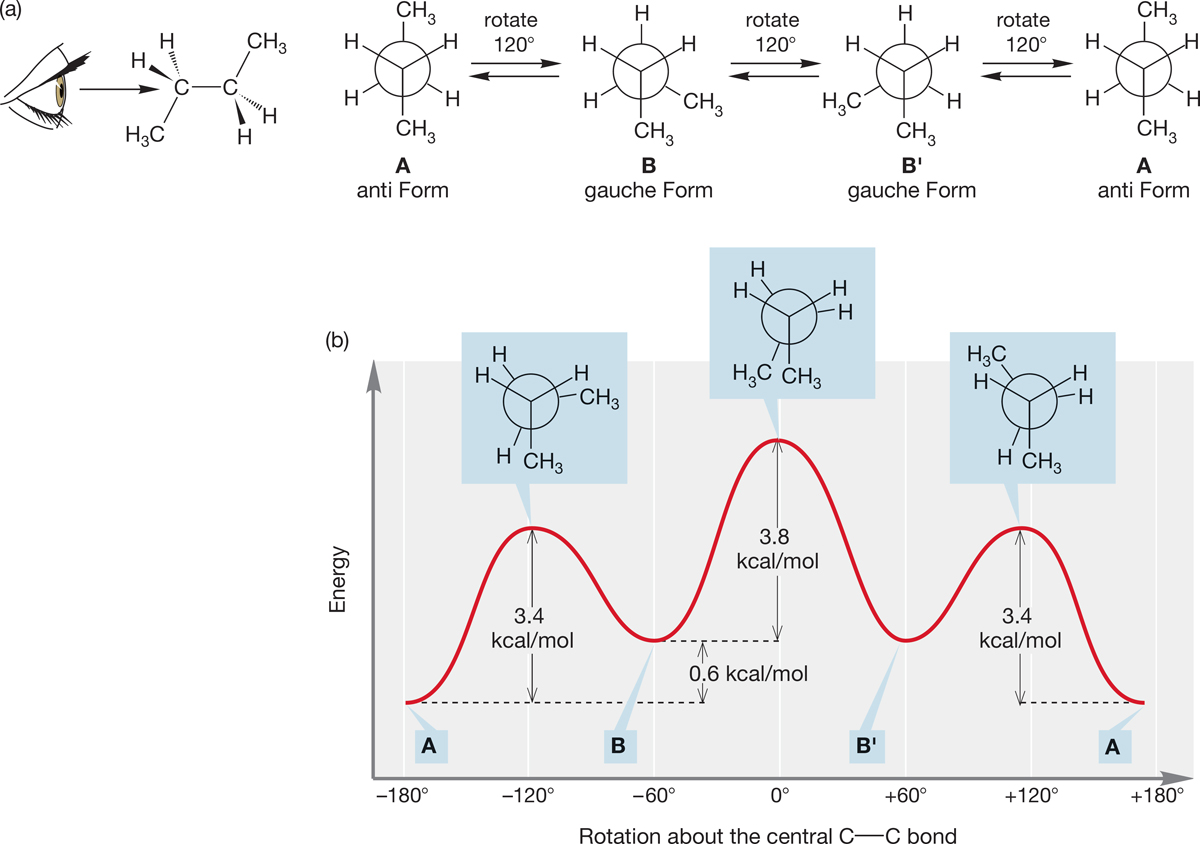
We can represent molecules in several ways, as summarized in Figures 2.38 and 2.39. The line drawings are the preferred choice for most cases. The convention of solid and dashed wedges gives more detail about the three-dimensional structures. Propane (C3H8) has staggered and eclipsed conformations, and the barrier between them is relatively low (3.4 kcal/mol). Two isomers are possible for substituted propane (C3H7―X), with the common names propyl and isopropyl.
Conformational analysis of butane (C4H10) introduces several new concepts. Butane has anti and gauche forms. The energy diagram for rotation around the central bond of butane (Fig. 2.33b) shows the effects of the steric interactions of eclipsing methyl groups. There are four isomers for the substituted butanes (C4H9―X), with the common names of butyl―X, sec-butyl―X, tert-butyl―X, and isobutyl―X.
Pentane has two structural isomers: isopentane and neopentane. There are eight isomers of the substituted pentanes (C5H11―X). A helpful tool for distinguishing between the different substituted compounds is the very important system of classifying carbons as primary, secondary, and tertiary.