2.8 Butanes (C4H10), Butyl Compounds (C4H9X), and Conformational Analysis
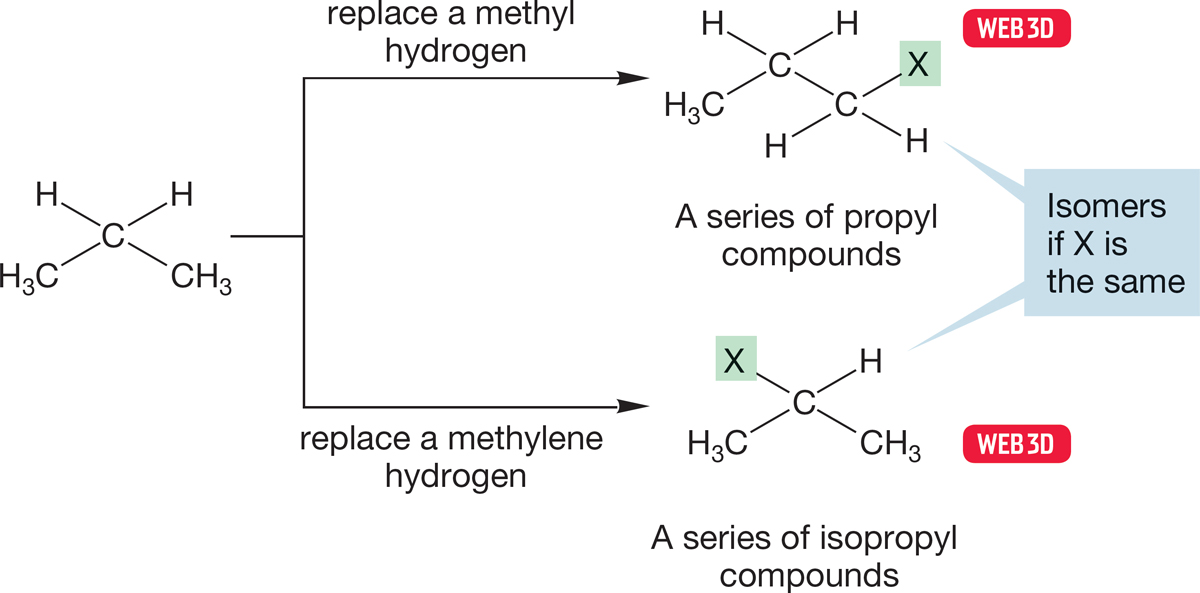
In Figure 2.30, if we let X be methyl in the two propyl isomers on the right, we generate the isomers butane and isobutane (Fig. 2.31).
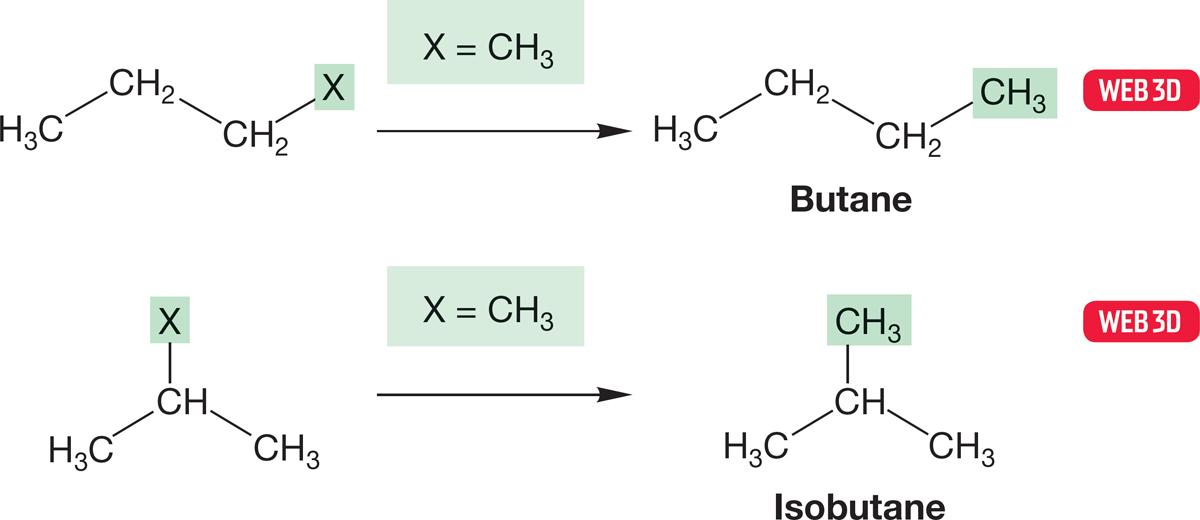
FIGURE 2.31 If X = CH3, we produce the isomers butane and isobutane.
The structure of butane (Fig. 2.32) provides a nice example of conformational analysis, the study of the relative energies of conformational isomers.
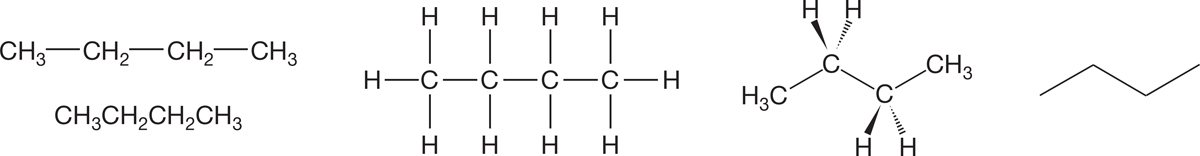
FIGURE 2.32 Different representations of butane.
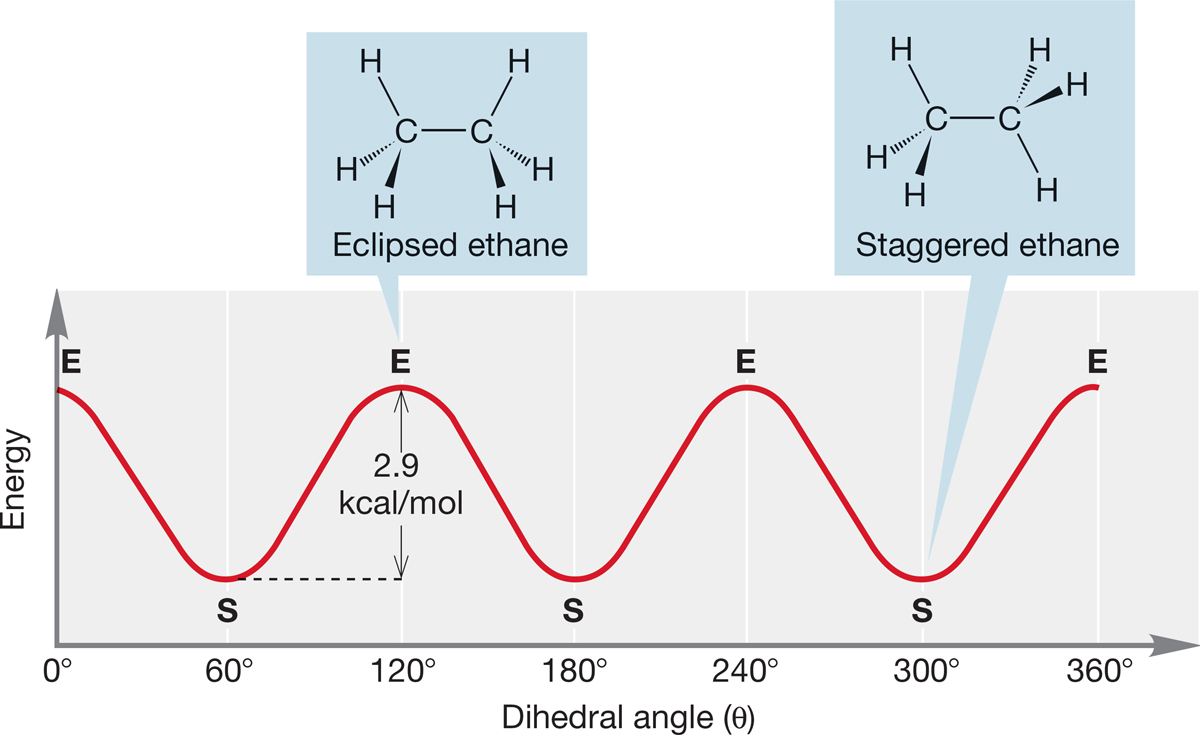
Let’s begin this analysis by constructing a Newman projection by looking down the C(2)―C(3) bond of butane (Fig. 2.33a). The notation C(2), C(3), and so on, will be used to refer to the carbon atom number. If we make the reasonable assumption that the relatively large methyl groups attached to these two carbons are best kept as far from each other as possible, we have the most stable staggered conformation A in Figure 2.33a, which is called the anti form. As in ethane (see Fig. 2.23), a 120° clockwise rotation of the C(2)―C(3) bond, keeping C(2) fixed, passes over a transition state (an energy maximum); this is the first transition state in Figure 2.33b. As the rotation continues, the molecule acquires a new staggered conformation, B. Conformation B is less stable than A because in B the two methyl groups are closer to each other than they are in A. Conformation B is called gauche-butane, and this kind of methyl–methyl interaction is a gauche interaction. The gauche form of butane (B) is about 0.6 kcal/mol (2.5 kJ/mol) higher in energy than the anti form (A).5 The eclipsed transition state for the interconversion of staggered conformation A and staggered conformation B lies about 3.4 kcal/mol (14.2 kJ/mol) higher in energy than A.
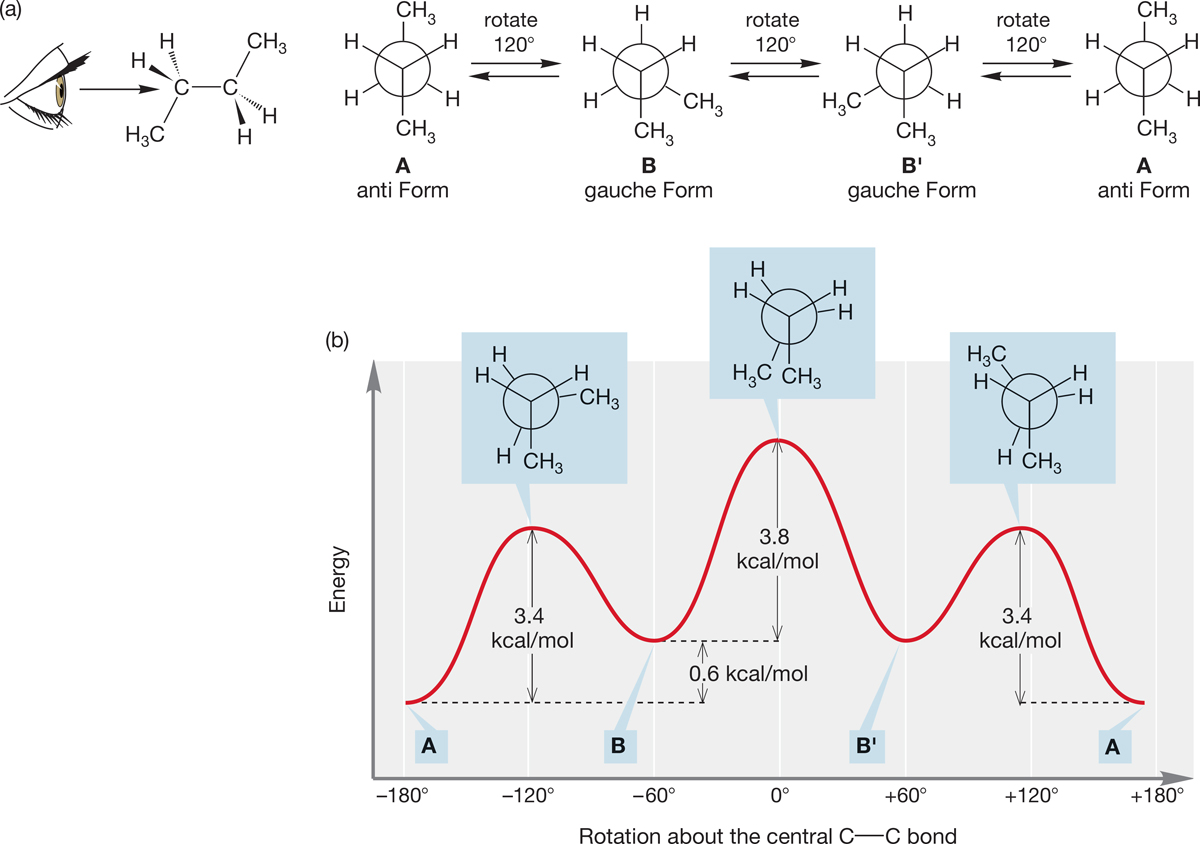
FIGURE 2.33 (a) Newman projections for butane. The staggered conformations are shown. (b) A graph of dihedral angle (θ) versus energy for butane as the molecule changes through rotation about the C(2)―C(3) bond. All three eclipsed conformations are shown.
Another 120° clockwise rotation brings us to a second gauche-butane, B′. The transition state for the conversion of B to B′, the second transition state in Figure 2.33b, involves an eclipsing of two C―CH3 bonds and lies 3.8 kcal/mol (15.9 kJ/mol) above B and B′, a total of about 4.4 kcal/mol (18.4 kJ/mol) above A. A final 120° clockwise rotation returns us to A.
PROBLEM 2.21 Recall from Figure 2.23 that the staggered ethane conformation is 2.9 kcal/mol more stable than its eclipsed conformation. Given that value and the information that the transition state for the interconversion of staggered conformations A and B in butane is 3.4 kcal/mol higher in energy than A (Fig. 2.33b), calculate how much energy each eclipsed C―CH3/C―H interaction in the transition state for the interconversion of A and B is worth. How much energy is the eclipsed C―CH3/C―CH3 interaction in the transition state for the conversion of B to B∙ worth?
PROBLEM 2.22 Draw Newman projections constructed by looking down the indicated carbon–carbon bond in the following molecules. For the molecule on the right, make a graph of energy versus dihedral angle.
We start our analysis of branched compounds with the butyl compounds, C4H9―X. Butane contains two kinds of hydrogen atoms in its pairs of equivalent methylene (CH2) groups and methyl (CH3) groups. Replacement of hydrogen a or b produces two types of butyl compounds, as shown in Figure 2.34a and b. Similarly, isobutane contains a single methine group (CH) and three equivalent methyl groups and also yields two types of butyl compounds (Fig. 2.34c and d).
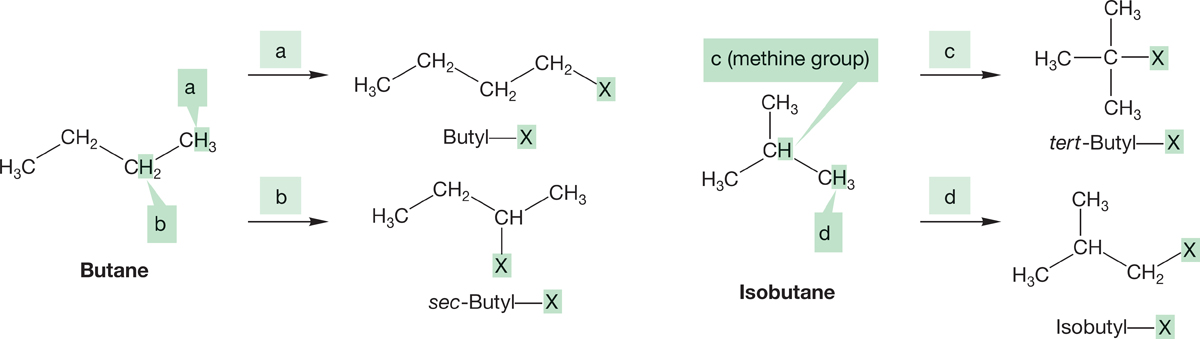
FIGURE 2.34 (a) and (b) Replacement of one of the hydrogens of butane with X yields two kinds of butyl derivatives, butyl―X and sec-butyl―X. (c) and (d) Replacement of hydrogen with X in isobutane yields two more butyl compounds, tert-butyl―X and isobutyl―X.
The four different types of butyl compounds are called butyl, isobutyl, sec-butyl (short for secondary-butyl), and tert-butyl (or sometimes t-butyl, short for tertiary-butyl). To see what the prefixes sec- and tert- mean, we must introduce new and important terminology for sp3-hybridized carbons (Fig. 2.35). A primary carbon is a carbon that is attached to only one other carbon atom. A secondary carbon is a carbon attached to two other carbons, and a tertiary carbon is a carbon attached to three other carbons. A quaternary (not quarternary) carbon (not shown in Figure 2.35, but we’ll see one in a moment) is a carbon that is attached to four other carbons. Thus, the names sec-butyl and tert-butyl tell you something about the structure.
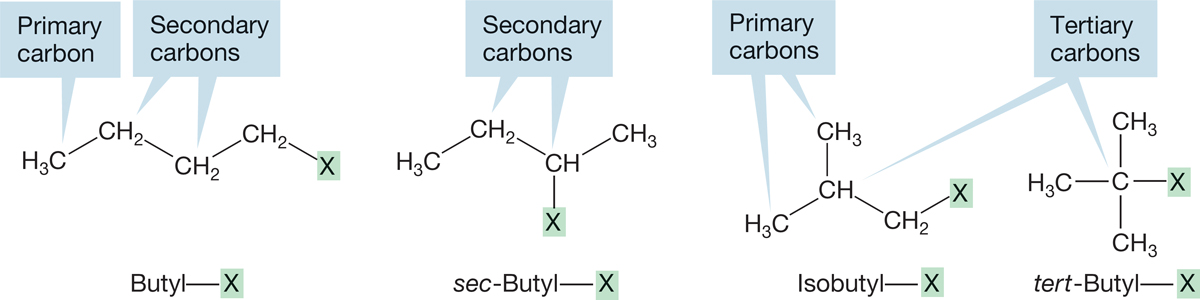
FIGURE 2.35 The four kinds of butyl compounds used to illustrate the bonding in primary, secondary, and tertiary carbons (X is not carbon).
PROBLEM 2.23 Draw two compounds containing quaternary carbons.
5This number, 0.6 kcal/mol, is somewhat controversial. Most books give this value as 0.9 kcal/mol. That 0.9 figure is correct for the gas phase but is not correct in solution. Indeed, the value is different in different solvents. It seemed best to give a number for solution, in which most chemistry takes place. You will find slight differences in almost every version of the curve in Figure 2.33, but the big picture—the general shape of the curve—will not vary. The reasons for the difference between the solution and the vapor phase are complex, but if you would like to read more, see E. E. Eliel and S. H. Wilen, Stereochemistry, pp. 600ff. (Wiley, New York, 1994).