6.2 Nomenclature of Substituted Alkanes
6.2a Alkyl Halides Alkyl iodides, bromides, chlorides, and fluorides are collected under the name of alkyl halides. Alkyl halides commonly have the formula CnH2n + 1X, where X = F, Cl, Br, or I. Quite appropriately, alkyl halides are named as fluorides, chlorides, bromides, and iodides. Both common and systematic nomenclature names are used, although as the complexity of the molecule increases, the system naturally takes over. The important rules to remember (p. 83) for naming saturated halides are (1) use the root word that corresponds to the longest carbon chain, (2) minimize the number given to the substituent, (3) name multiple substituents in alphabetical order, and (4) if numbering the chain results in the same substituent numbers from either end, give the priority to the alphabetical order. If a carbon–carbon double bond is present, the double bond takes precedence over the halogen when positional numbers are assigned. Table 6.1 lists some examples with both common and systematic names given. Some important common names are used very often. For example, haloforms are CHX3, vinyl halides are H2C CHX, and allyl halides are H2C
CHX, and allyl halides are H2C CH―CH2X.
CH―CH2X.
TABLE 6.1 Some Alkyl Halides, Names, and Known Properties
Compound |
Common Name |
Systematic Name |
bp (ºC) |
mp (ºC) |
|
Methyl fluoride |
Fluoromethane |
–78.4 |
–141.8 |
|
Ethyl bromide |
Bromoethane |
38.4 |
–118.6 |
|
Isopropyl iodide |
2-Iodopropane |
89.4 |
–90.1 |
|
tert-Butyl chloride |
2-Chloro-2-methylpropane |
51.0 |
–25.4 |
|
Methylene bromide |
Dibromomethane |
97 |
–52.7 |
|
Chloroform |
Trichloromethane |
61.5 |
–63.5 |
|
Vinyl bromide |
Bromoethene |
15.8 |
–139.5 |
|
Allyl chloride |
3-Chloropropene |
45 |
–134.5 |
|
|
2-Bromo-1-chlorobutane |
147 |
|
|
|
3-Chloro-1-pentene |
94 |
|
|
|
Bromocyclopentane |
138 |
|
|
||||
bp, boiling point; mp, melting point.
Halides are classified as methyl, primary, secondary, tertiary, and vinyl (Fig. 6.2). We introduced these descriptions in Chapter 2 (p. 79). Remember: A primary carbon is attached to only one other carbon, a secondary carbon to two others, and a tertiary carbon to three others. It is important to note that methyl, primary, secondary, and tertiary carbons are sp3 hybridized. A carbon in a double bond (a vinyl carbon) is never called primary, secondary, or tertiary.
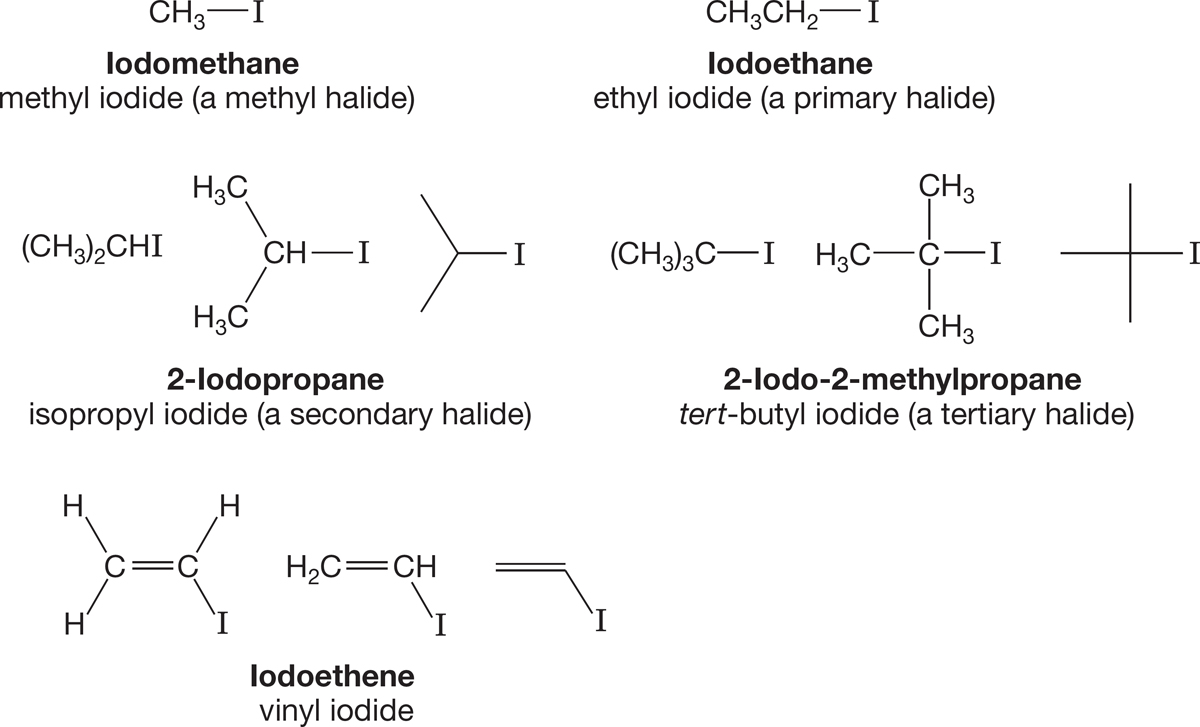
FIGURE 6.2 Some alkyl and vinyl halides represented as condensed, full, or line structures.
In a primary halide, the halogen atom is attached to a primary carbon, and so on. In vinyl halides, there is always a carbon–carbon double bond to which the halide is directly attached.
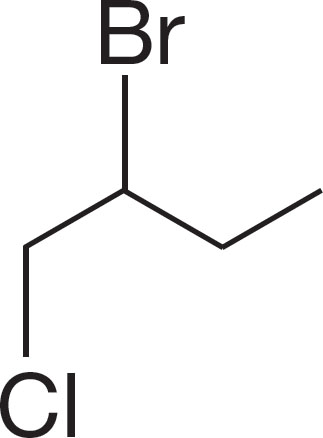
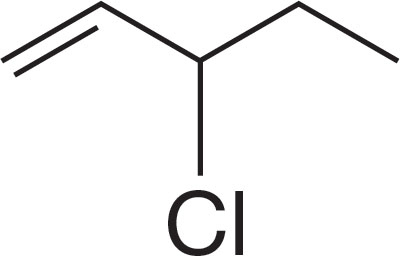
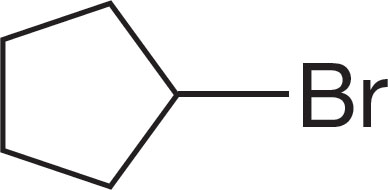
PROBLEM 6.1 Name the following compounds:
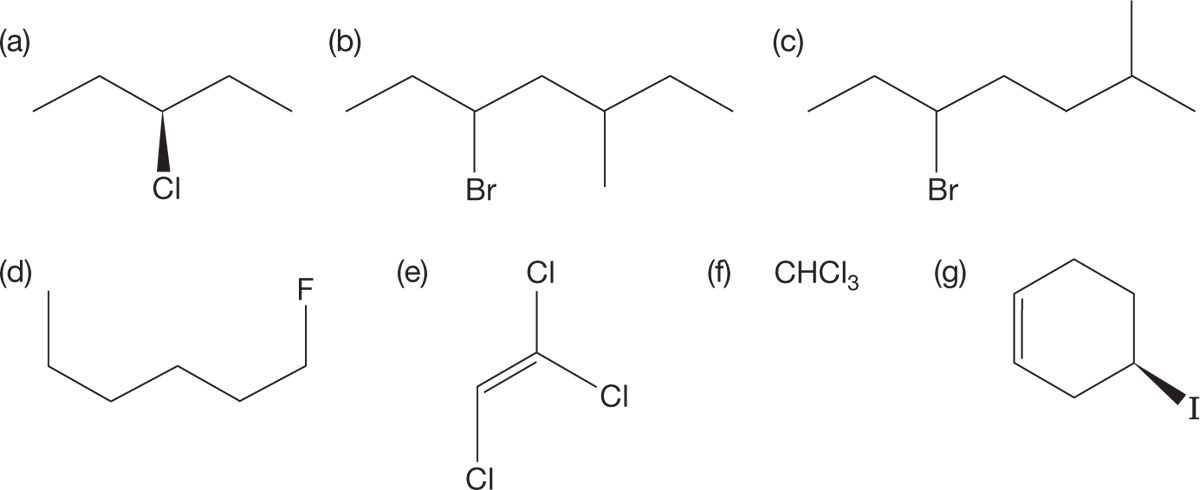
PROBLEM 6.2 Draw structures for the following compounds:
(a) (3R,4S)-dichlorohexane
(b) 3-bromocyclohexene
(c) (Z)-1-bromo-1-butene
(d) iodoethene
(e) 1,1-dichlorocyclopropane
6.2b Alcohols There are two ways to name alcohols: the International Union of Pure and Applied Chemistry (IUPAC) systematic way and the way chemists often do it, by using the splendidly vulgar common names. As usual, these common names are used only for the smaller members of the class, and the system takes over as molecular complexity increases.
Alcohols are named systematically by (1) finding the longest chain that includes the carbon attached to the OH group, (2) dropping the final “e” of the parent hydrocarbon name and adding the suffix “ol,” (3) giving the OH functional group the lowest number possible because it has priority over the other functional groups we have learned so far (see the inside front cover of the book) and, (4) alphabetically arranging the substituents in the prefix (Fig. 6.3).
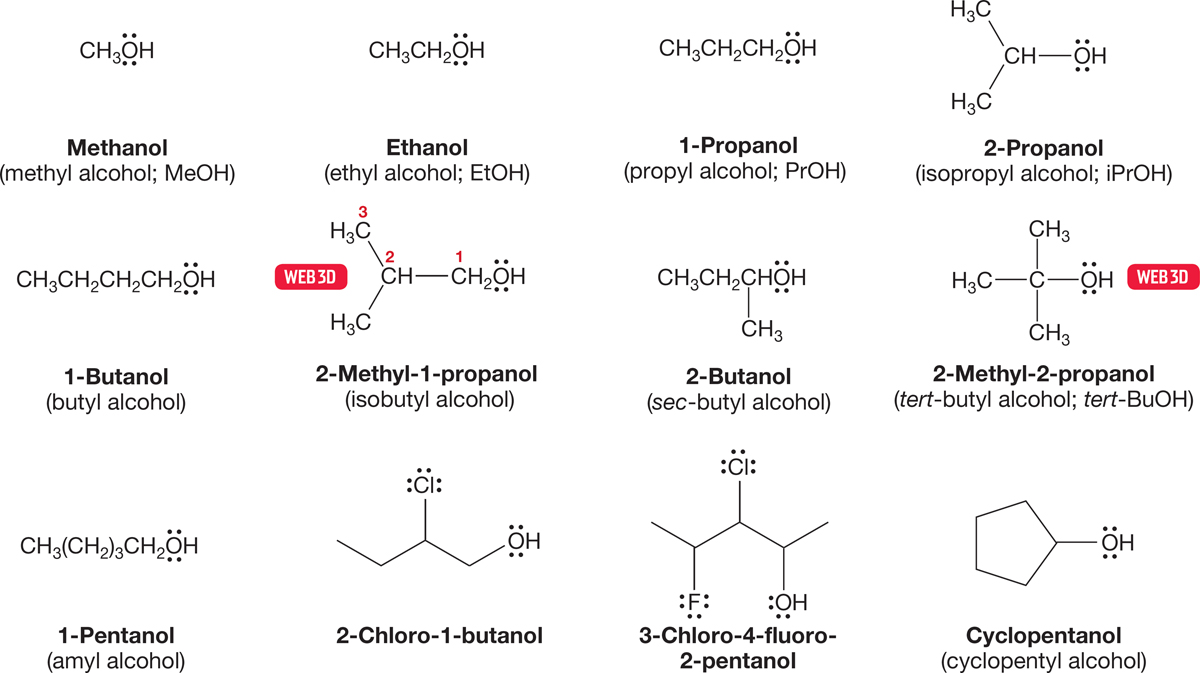
FIGURE 6.3 Some systematic and commonly used names for alcohols.
The smaller alcohols have common names with the word alcohol written after the appropriate group name. Figure 6.3 gives some systematic and common names for the smaller alcohols. When we get past approximately five carbons, the systematic naming protocol takes over completely, and even the delightful name amyl for the five-carbon alcohols is disappearing.1 However, some common names seem to be quite hardy. For example, the correct IUPAC name for hydroxybenzene is benzenol, but the common name phenol is still accepted in the IUPAC system and probably will survive for a long time (Fig. 6.4).
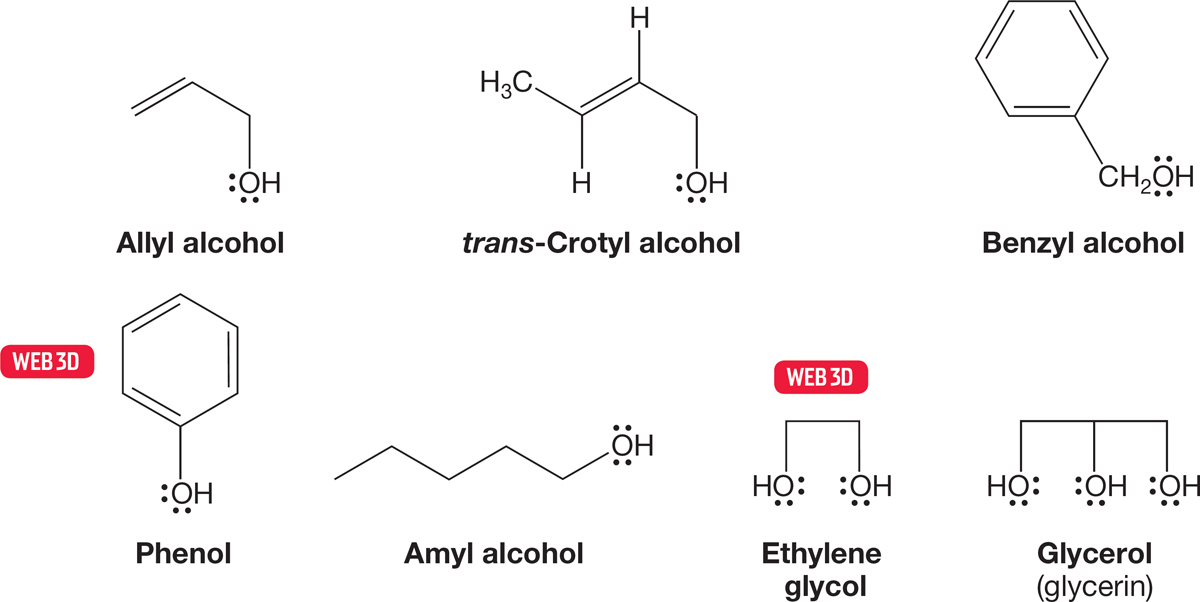
FIGURE 6.4 Some widely used common names for alcohols.
PROBLEM 6.3 Provide systematic names for the following alcohols:
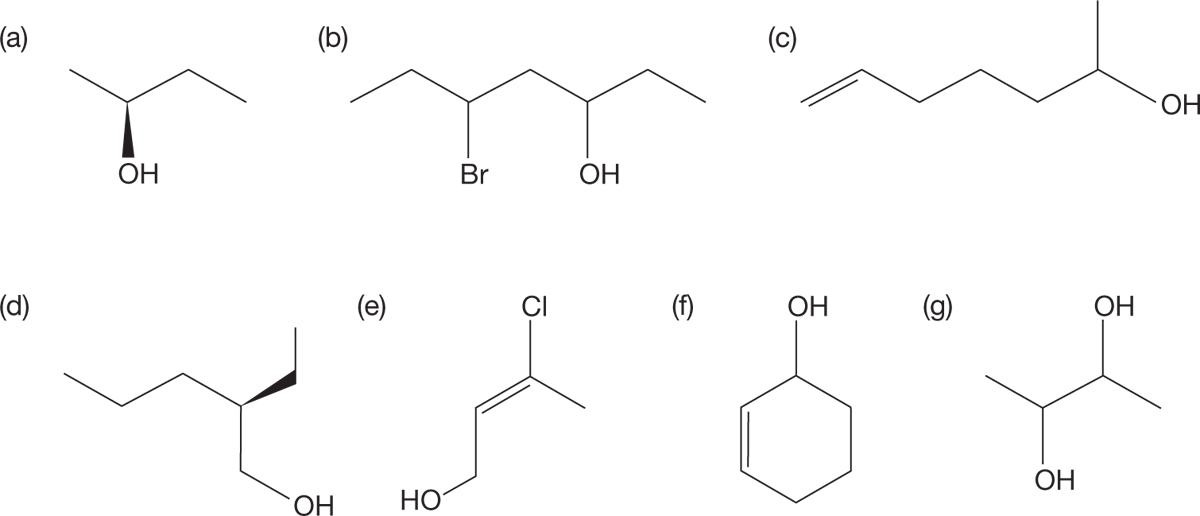
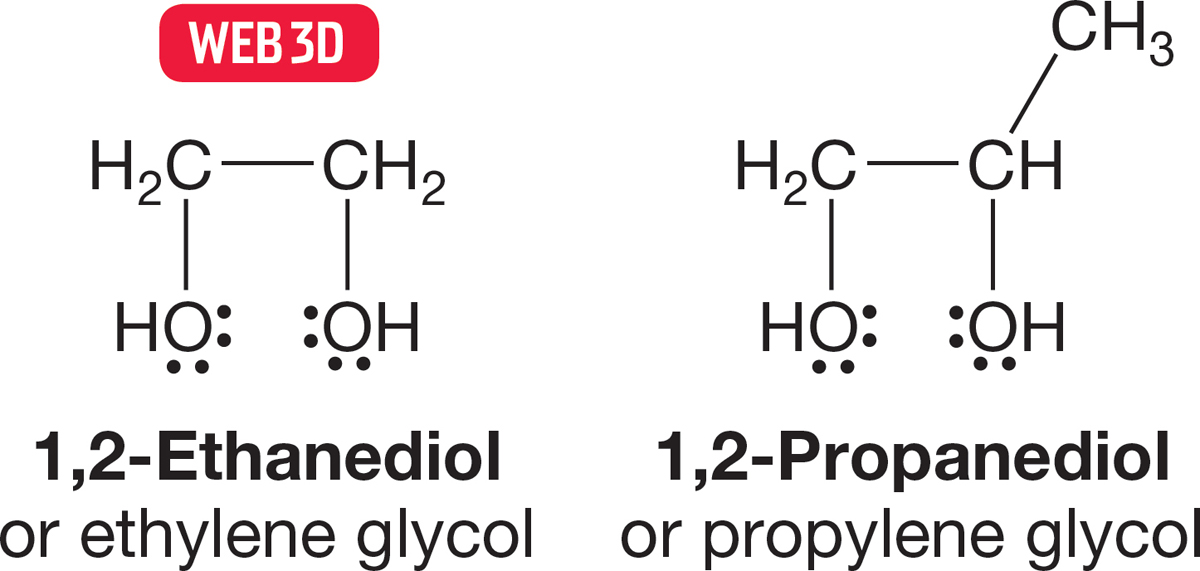
FIGURE 6.5 1,2-Ethanediol and 1,2-propanediol are called ethylene glycol and propylene glycol, respectively.
Although molecules containing two OH groups are logically enough called diols, there is an often-used common name as well: glycol. Some 1,2-diols or 1,2-glycols are familiar and are often important molecules. 1,2-Ethanediol, or ethylene glycol, is a commonly used antifreeze in cars, for example. Note that in naming these compounds, the final “e” of the alkane parent compound is not dropped, as it is in naming simple alcohols, and that the almost universally used common name can be very misleading. Ethylene glycol implies the presence of an alkene, and propylene glycol (1,2-propanediol) suggests a three-carbon unsaturated chain. In neither case is the implied unsaturation present (Fig. 6.5). Be aware that the real structures are based on saturated hydrocarbon chains.
6.2c Amines We first saw amines in Chapter 1, albeit very briefly. Amines are derivatives of ammonia, :NH3 (Fig. 6.6). Successive replacement of the hydrogens of ammonia leads to primary amines, secondary amines, and tertiary amines, :NH2R, :NHR2, and :NR3 respectively.
Quaternary nitrogen compounds are positively charged and are called ammonium ions. Many systems for naming amines exist, and the chemical world seems to be resisting attempts to bring order out of this minichaos by keeping to the old common names.

FIGURE 6.6 Substituted amines.
Primary amines are commonly named by using the name of the substituent, R, and appending the suffix “amine.” Secondary and tertiary amines in which the R groups are all the same are simply named as dialkylamines and trialkylamines. Amines bearing different R groups are named by ordering the groups alphabetically. Ethylmethylamine is correct, methylethylamine is not, although both are surely intelligible (Fig. 6.7).
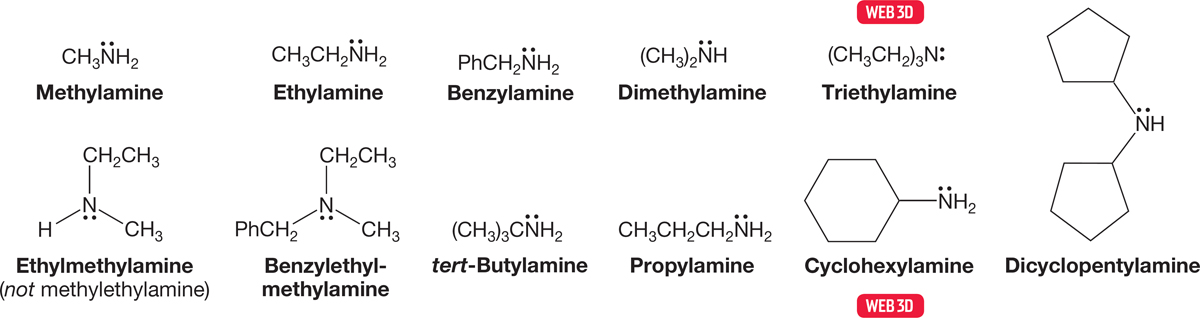
FIGURE 6.7 A naming scheme for primary, secondary, and tertiary amines.
The IUPAC system names amines analogously to alcohols. If CH3OH is officially known as methanol, then CH3NH2 is methanamine. The same steps that were used to name alcohols can be used for naming amines. The steps are (1) find the longest carbon chain with the carbon attached to a nitrogen and use that parent hydrocarbon name, (2) drop the final “e” of the parent hydrocarbon and add the suffix “amine,” (3) number the chain so that the priority group, the amine in this case, has the lowest possible number, and (4) alphabetically arrange the substituents in the prefix. Secondary and tertiary amines will have the alkyl groups listed in alphabetical order in the prefix as N-alkyl or N,N-dialkyl if the groups are the same. Figure 6.8 gives several examples.
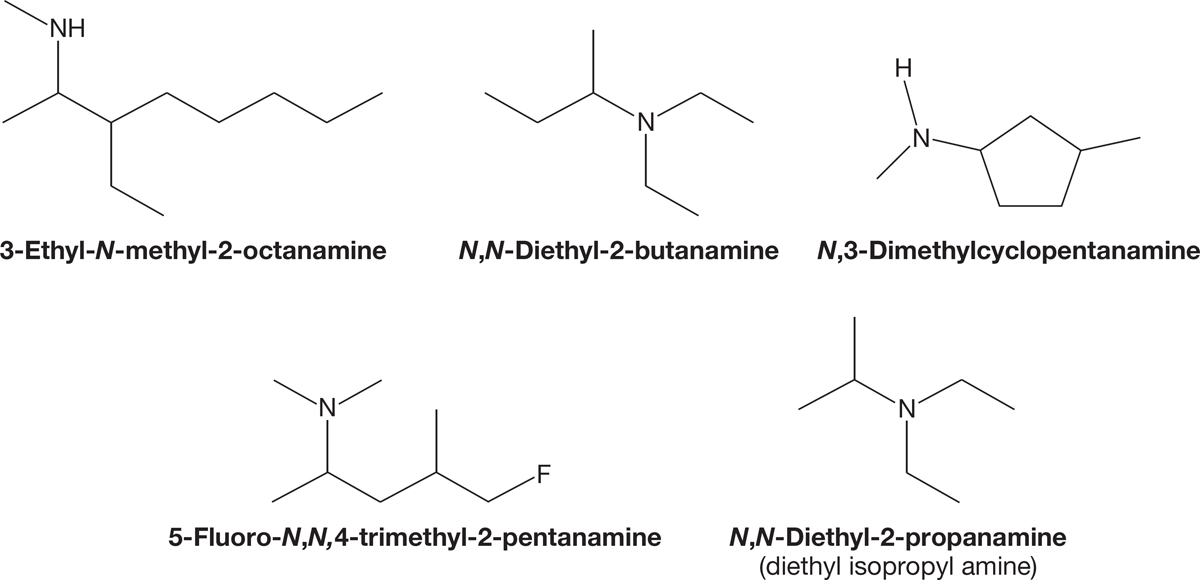
FIGURE 6.8 Systematically named amines.
The IUPAC priority system (see the inside front cover of the book) has the alcohol group with a higher priority than the amine group. So a molecule that contains both an alcohol and an amine group will be named as the alcohol (Fig. 6.9), and the amine is listed as an amino group in the prefix.
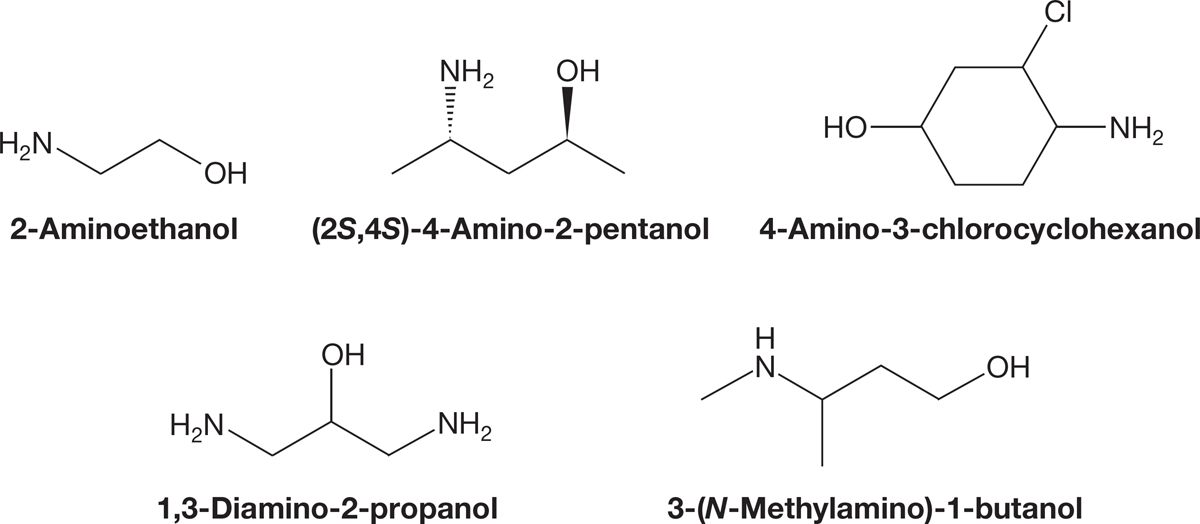
FIGURE 6.9 The use of “amino” as a prefix when naming molecules with a higher-priority group.
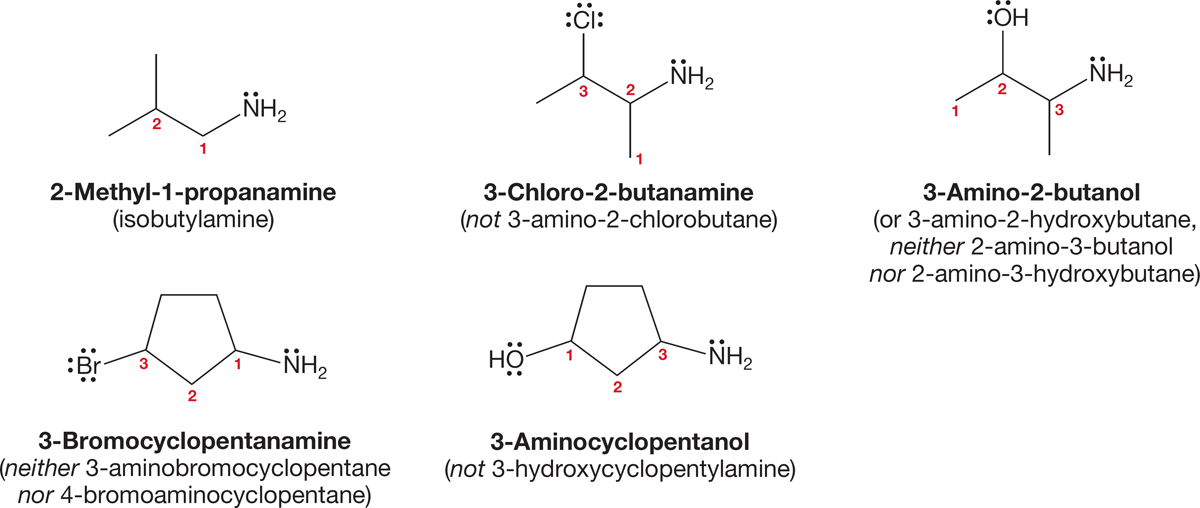
FIGURE 6.10 Some polyfunctional amines and their names.
Figure 6.10 gives some correct and incorrect names of polyfunctional compounds containing amino groups.
Simple, cyclic amino compounds are also widely seen in nature and are almost invariably known by their common names. These compounds are often named as “aza” analogues of an all-carbon system. Thus aziridine becomes azacyclopropane. Unless there is an oxygen atom in the ring, the numbering scheme assigns the nitrogen atom as number 1 and proceeds so as to minimize the numbering of any substituents. Figure 6.11 gives a few important examples.
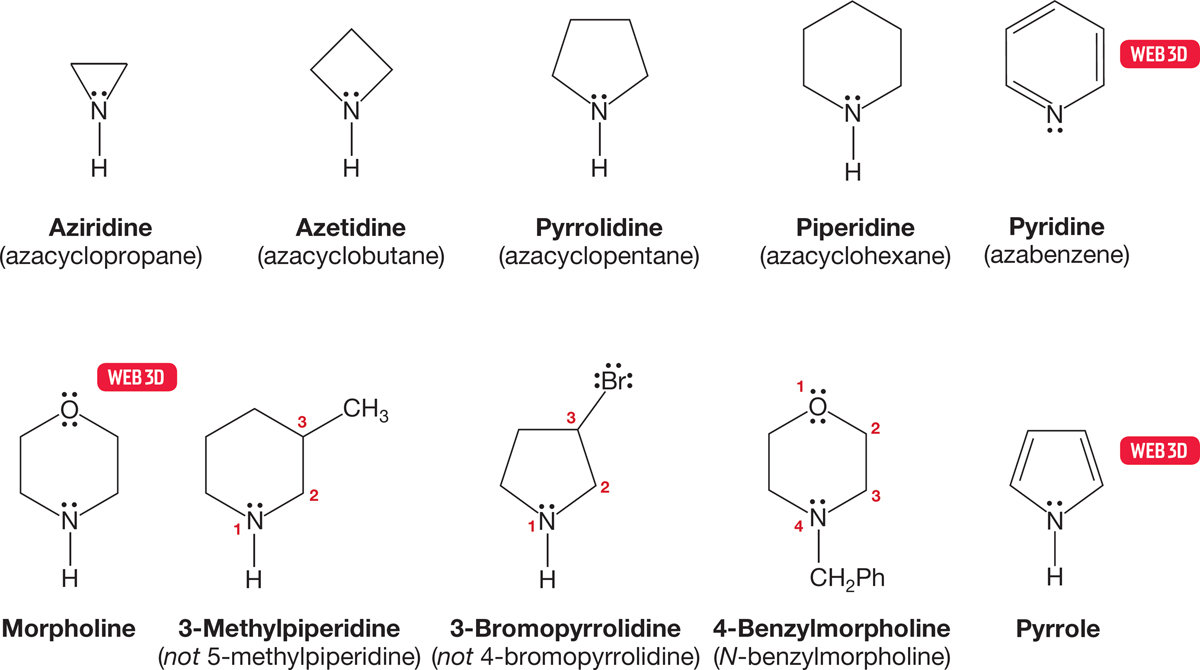
FIGURE 6.11 Cyclic amines and their names.
Ammonium ions are named by alphabetically attaching the names of the substituents. Don’t forget to append the name of the negatively charged counterion (Fig. 6.12).
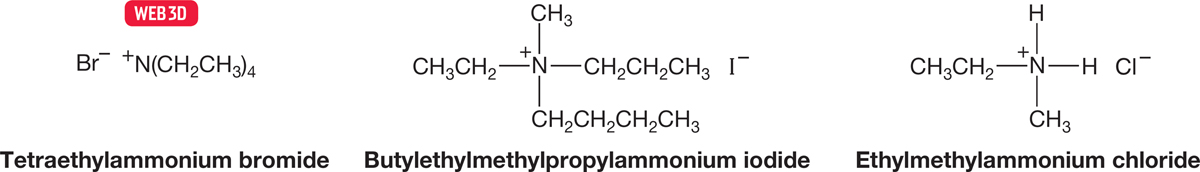
FIGURE 6.12 Names for three ammonium ions.
6.2d Ethers Compounds that have the structure R―O―R or R―O―R′ are called ethers. Naming ethers is simple. In the common system, the two alkyl groups joined by oxygen are named in alphabetical order, and the word ether is appended. In the IUPAC system, ethers are named as alkoxy (RO) alkanes, and groups are listed in the prefix in alphabetical order. An ether is a subordinate group (see the inside front cover of the book) like the halides, which means in the IUPAC system it can only be named as a prefix. Figure 6.13 gives some simple examples of both usages. The IUPAC names are listed first.
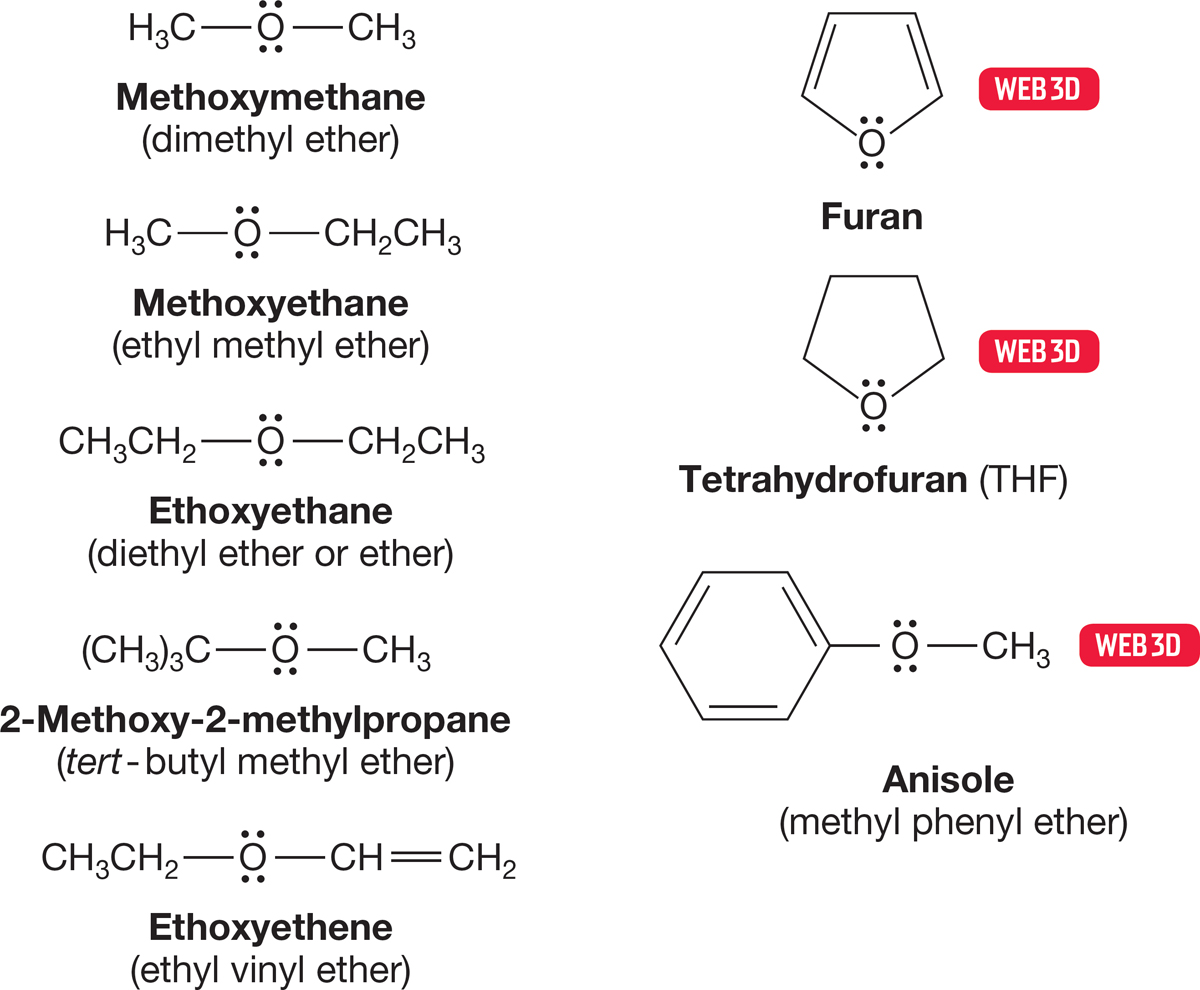
FIGURE 6.13 Some ethers and their common names.
6.2e Cyclic Ethers Cyclic ethers are named either as “oxa” ring compounds or as oxides by counting the number of methylene groups in the ring. Thus, cyclopentane with an oxygen in the ring becomes either oxacyclopentane or tetramethylene oxide. However, be careful—cyclic ethers are also named “commonly.” There is a substantial number of such names and they are generally the ones used. For example, oxacyclopentane is almost always known as tetrahydrofuran (THF). Figure 6.14 gives a sample of cyclic ethers named both systematically and commonly.
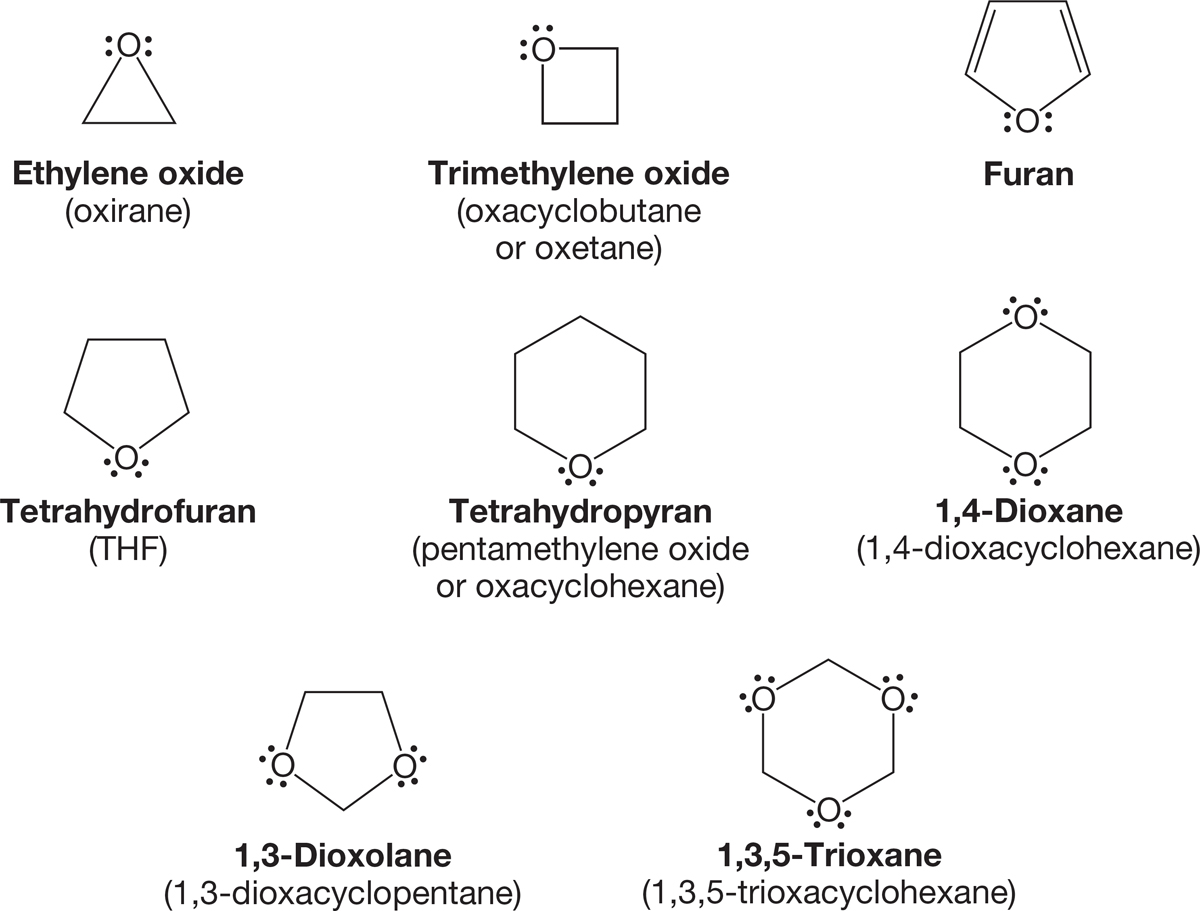
FIGURE 6.14 Some cyclic ethers and polyethers.
The three-membered cyclic ether deserves special discussion because it is frequently encountered. The three-membered ring containing an oxygen has the common name of epoxide. We often use this kind of molecule both in organic chemistry and regularly in everyday life. For example, it is one of the substances used in epoxy glues.
There are three methods you should be comfortable using for naming epoxides. The most versatile IUPAC method treats the epoxide as a substituent. It has you find the longest carbon chain and then use the term “epoxy” in the prefix for the two carbons attached to the ring oxygen (Fig. 6.15). Like all ethers, the epoxy group is a subordinate group. So the two carbons involved in the epoxide and the epoxy descriptor are placed alphabetically in the prefix.
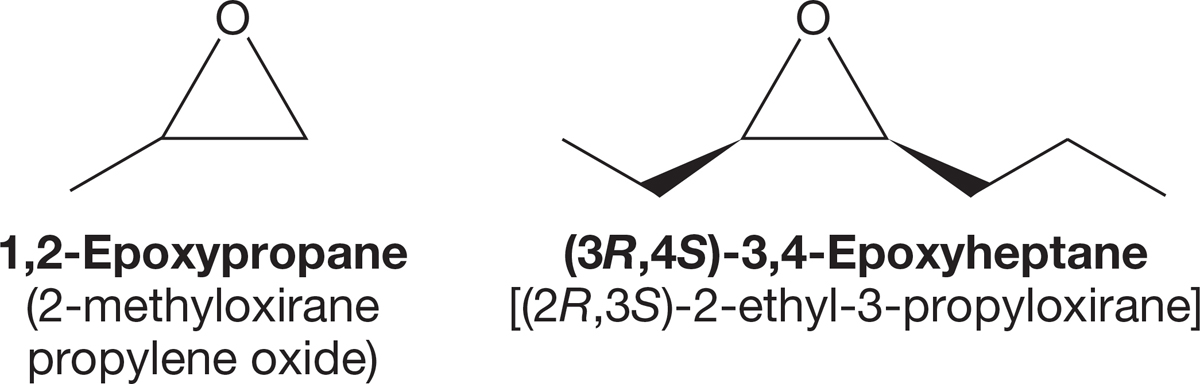
FIGURE 6.15 Naming epoxides.
A second method uses the IUPAC term oxirane as the root word. This is somewhat limiting, because the groups attached to the oxirane might be too complex to name as substituents. When using this method, the oxygen of the oxirane is atom number 1. The third method simply applies the term “oxide” to the common name of the parent alkene. This method is only used when there is a common name for the corresponding alkene. For example, the simplest oxirane is called ethylene oxide.
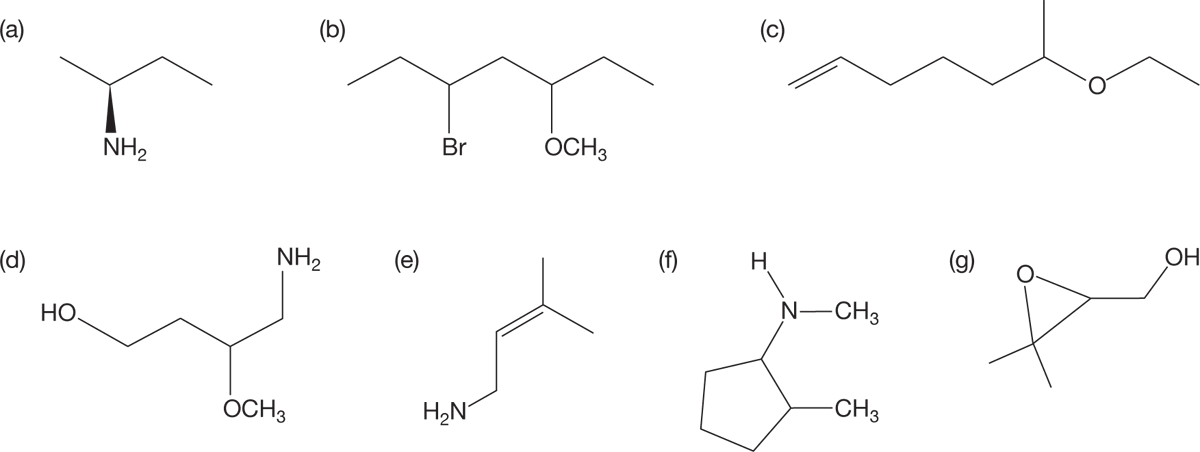
PROBLEM 6.4 Provide systematic names for the following amines and ethers:
1Amyl derives from the Latin word for “starch,” amylum. The first amyl alcohol was isolated from the fermentation of potatoes.
 CH3 ―F
CH3 ―F