6.5 Solubility
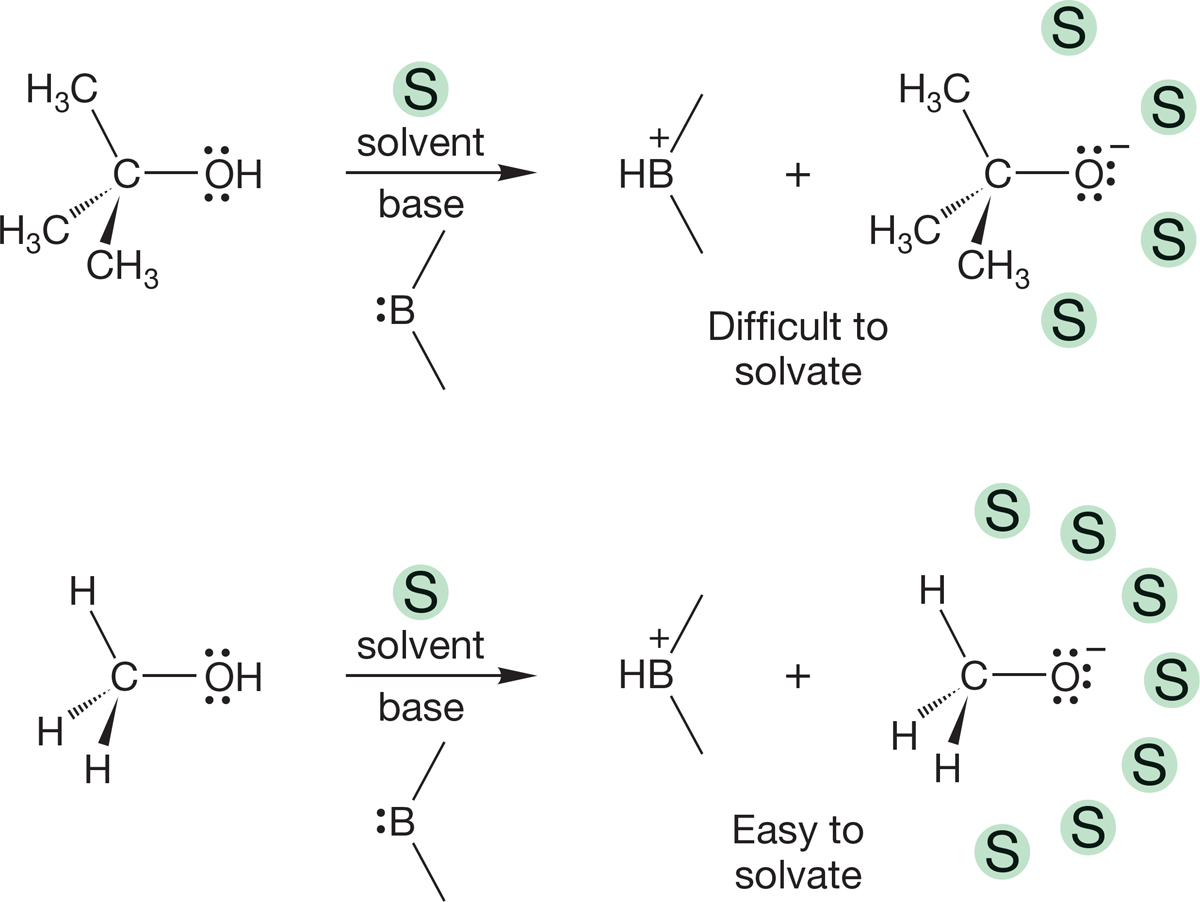
We have seen an example of a solvent playing a crucial role in determining the acidities of alcohols and amines. The greater acidity of methyl alcohol than tert-butyl alcohol in a polar solvent is a result of the greater ease of solvating the conjugate base of methyl alcohol―the methoxide ion―than the tert-butoxide ion (see Fig. 6.32). Let’s take a quick look at the properties of solvents and the process of solvation.
6.5a Polar Solvents We have seen that one kind of stabilization by solvent—solvation—is a consequence of hydrogen bonding. For hydrogen bonding to be possible, there clearly must be both a proton donor (usually a hydroxy or amino group, OH or NH2) and a proton acceptor (a pair of electrons) available. Solvents that can donate a proton are called protic solvents. Protic solvents are also usually quite polar; that is, they have relatively high dielectric constants, ε, a measure of the ability of a solvent to separate charges. Solvents without available protons—in other words, solvents that are not proton donors—are aprotic solvents and can be either polar or nonpolar. It is distinctly possible for a solvent to be quite polar, to have a high ε, but not be a proton donor. Many molecules are both nonpolar and aprotic; hydrocarbons are obvious examples that you know. Table 6.10 collects a few common polar solvents and their descriptions.
TABLE 6.10 Some Common Polar Solvents
Compound |
Name |
Dielectric Constant (ε) |
Description |
H2O |
Water |
78 |
Protic, polar |
HCOOH |
Formic acid |
59 |
Protic, polar |
CH3OH |
Methyl alcohol |
33 |
Protic, polar |
CH3CH2OH |
Ethyl alcohol |
25 |
Protic, polar |
(CH3)2SO |
Dimethyl sulfoxide (DMSO) |
47 |
Aprotic, polar |
CH3CN |
Acetonitrile |
38 |
Aprotic, polar |
(CH3)2NCHO |
Dimethylformamide (DMF) |
37 |
Aprotic, polar |
CH3NO2 |
Nitromethane |
36 |
Aprotic, polar |
|
|||
FIGURE 6.48 Two very different solids that dissolve easily in water.
6.5b Solubility: “Like Dissolves Like” Oil spreads on water, but very little dissolves. Two solids, salt (the prototypal ionic solid sodium chloride) and the sugar sucrose (C12H22O11, an organic material bristling with OH groups), both dissolve completely in water (Fig. 6.48). What is going on?
Sodium chloride, Na+Cl−, dissolves in the polar solvent water because both the positive sodium ion and the negative chloride ion are well solvated by the protic, polar solvent water (Fig. 6.49). Nonpolar solvents cannot solvate ions efficiently and do not dissolve sodium chloride.
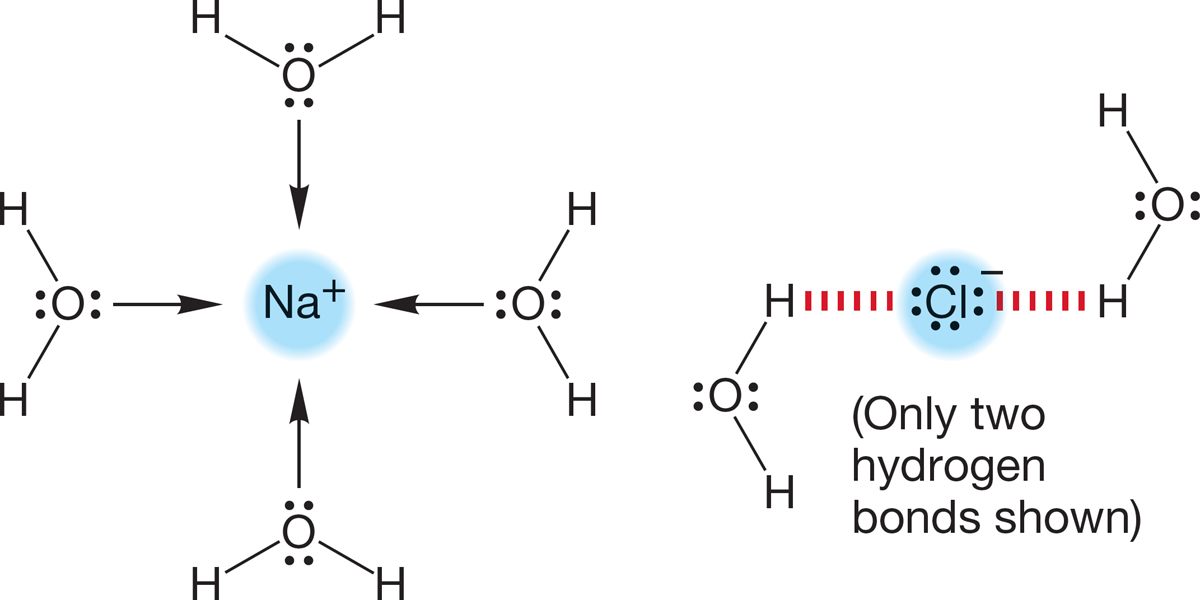
FIGURE 6.49 Solvation of the positive sodium cation and hydrogen bonding to the negative chloride ion (only two hydrogen bonds shown).
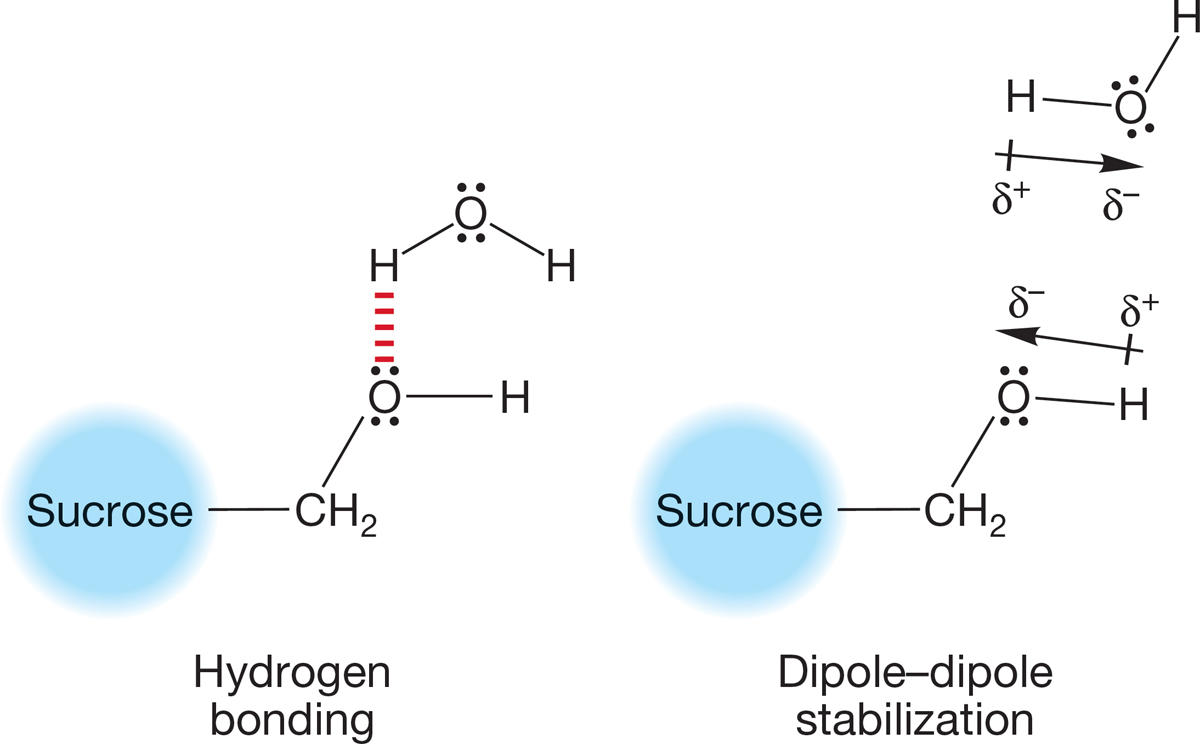
FIGURE 6.50 Solvation of an O―H bond by water.
Protic solvents are particularly good at dissolving molecules capable of hydrogen bonding. Formation of hydrogen bonds in solution is highly stabilizing. Thus sucrose, a molecule with many OH groups, dissolves easily in water. The polar solvent water stabilizes sucrose both through formation of many hydrogen bonds and through stabilization of the polarized O―H groups of sucrose. Figure 6.50 shows a schematic picture of hydrogen bonding (only one of many shown) and dipolar stabilization of the polyhydroxy compound sucrose by water.
Polar aprotic solvents can help disperse the partial charges in other polar aprotic molecules and thus dissolve them well. Although they cannot act as proton donors, such solvents can align their negative ends toward the positive charges in a polar molecule and their positive ends toward the negative charges. For example, Figure 6.51 shows the polar solvent THF solvating a water molecule by aligning dipoles in a stabilizing way. Water and THF are miscible.
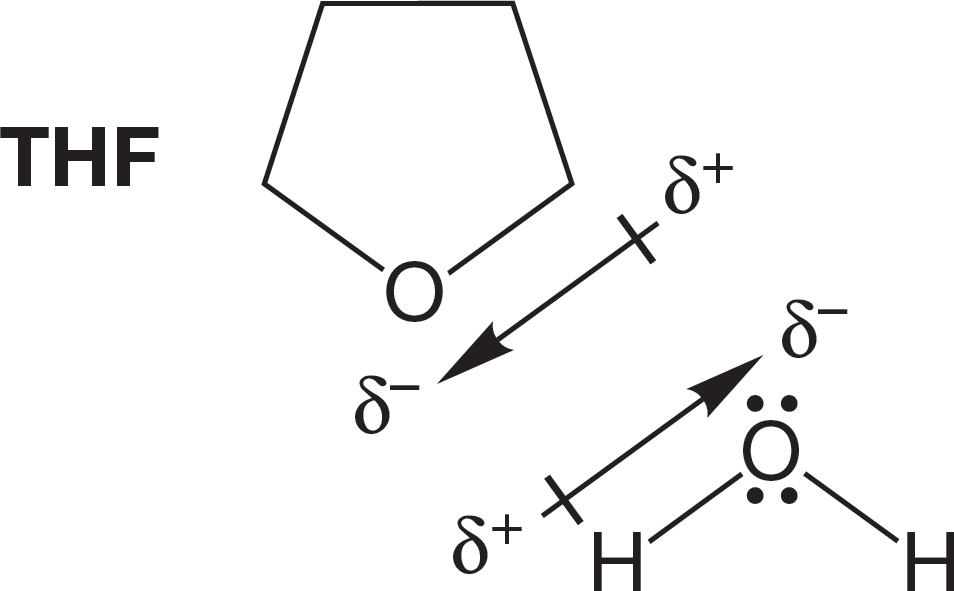
FIGURE 6.51 Stabilization (solvation) of water by tetrahydrofuran, THF. THF will also hydrogen bond with water.
By contrast, hydrocarbons cannot form hydrogen bonds (they are neither proton donors nor acceptors) and are not very polar. Typical ε values for hydrocarbons are around 2. The more nonpolar and “greasy” the solvent, the more easily the similarly nonpolar hydrocarbon molecules dissolve in it. Thus, hydrocarbons (oil is a mixture of many hydrocarbons) are almost completely insoluble in water but very soluble in other hydrocarbons. The polar molecule acetone [dimethyl ketone, (CH3)2CO], with an ε value of 21, is completely soluble in water, but the alkene isobutene, with the same number of nonhydrogen atoms, is almost completely insoluble in water. Like does dissolve like.
Summary
In the practical world of making chemical reactions work, we need to allow molecules to “find each other” and react. Dissolving molecules in a fluid medium provides an opportunity for molecules to move around and do just that—find other reactive molecules. The choice of solvent is critical because we need to dissolve the molecules we want to react together. In making that choice, the rather simple notion that “like dissolves like” really works. As we work through chemical reactions in the next few chapters, we will have to take account of solvents and solvation.