6.11 Additional Problems
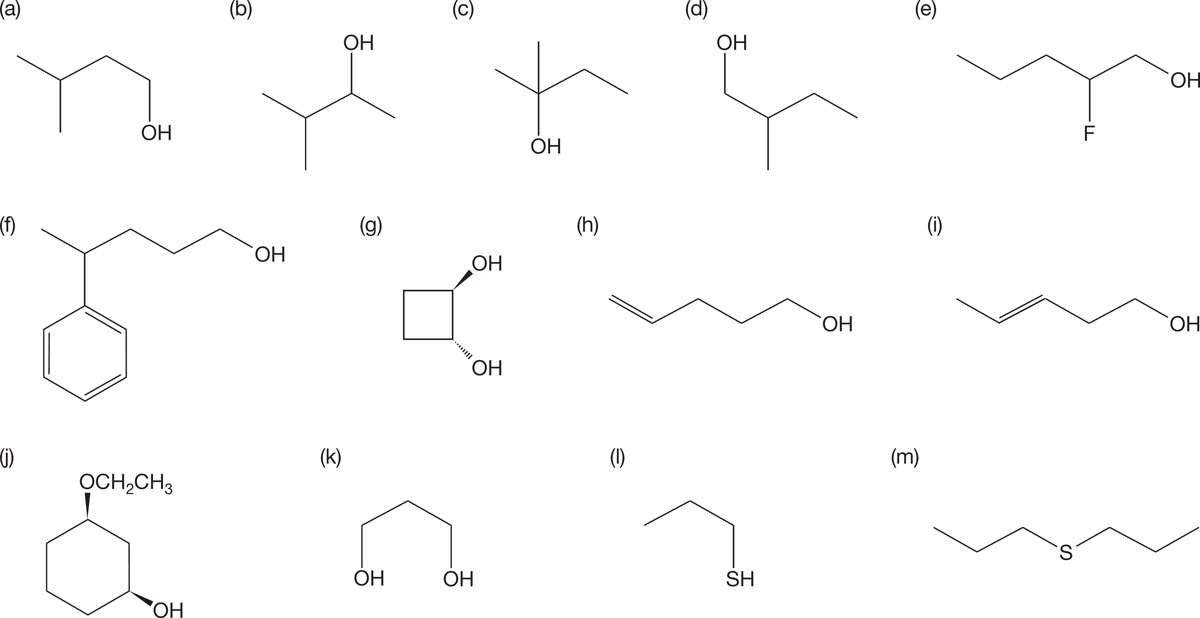
PROBLEM 6.21 Name the following compounds:
PROBLEM 6.22 Draw structures for the following compounds:
(a) 1,4-butanediol
(b) 3-amino-1-pentanol
(c) cis-4-tert-butoxycyclohexanol
(d) 2,2,4,4-tetramethyl-3-pentanol
(e) (R)-sec-butyl alcohol
(f) trans-3-chlorocyclohexanol
(g) tert-butyl cyclopentyl ether
PROBLEM 6.23 Draw structures for the following compounds:
(a) tert-butyl alcohol
(b) 2,3-dimethoxybutane
(c) cis-2,3-epoxyhexane
(d) THF
(e) ethylene oxide
PROBLEM 6.24 Draw structures for the following compounds:
(a) (R)-3-methyl-2-butanamine
(b) cis-2-ethylcyclopentanamine
(c) 1-hexanamine
(d) 3-methoxy-2-heptanamine
(e) N-methyl-3-hexanamine
(f) (Z)-2-penten-2-amine
PROBLEM 6.25 Draw structures for the following compounds:
(a) 4-chloro-N-ethyl-2-butanamine
(b) (2R,3R)-N-ethyl-2-methoxy-N-methyl-3-hexanamine
(c) trans-2,3-epoxy-1-ethoxybutane
(d) cis-3-aminocyclopentanol
(e) (2R,4S)-4-chloro-N-phenyl-2-heptanamine
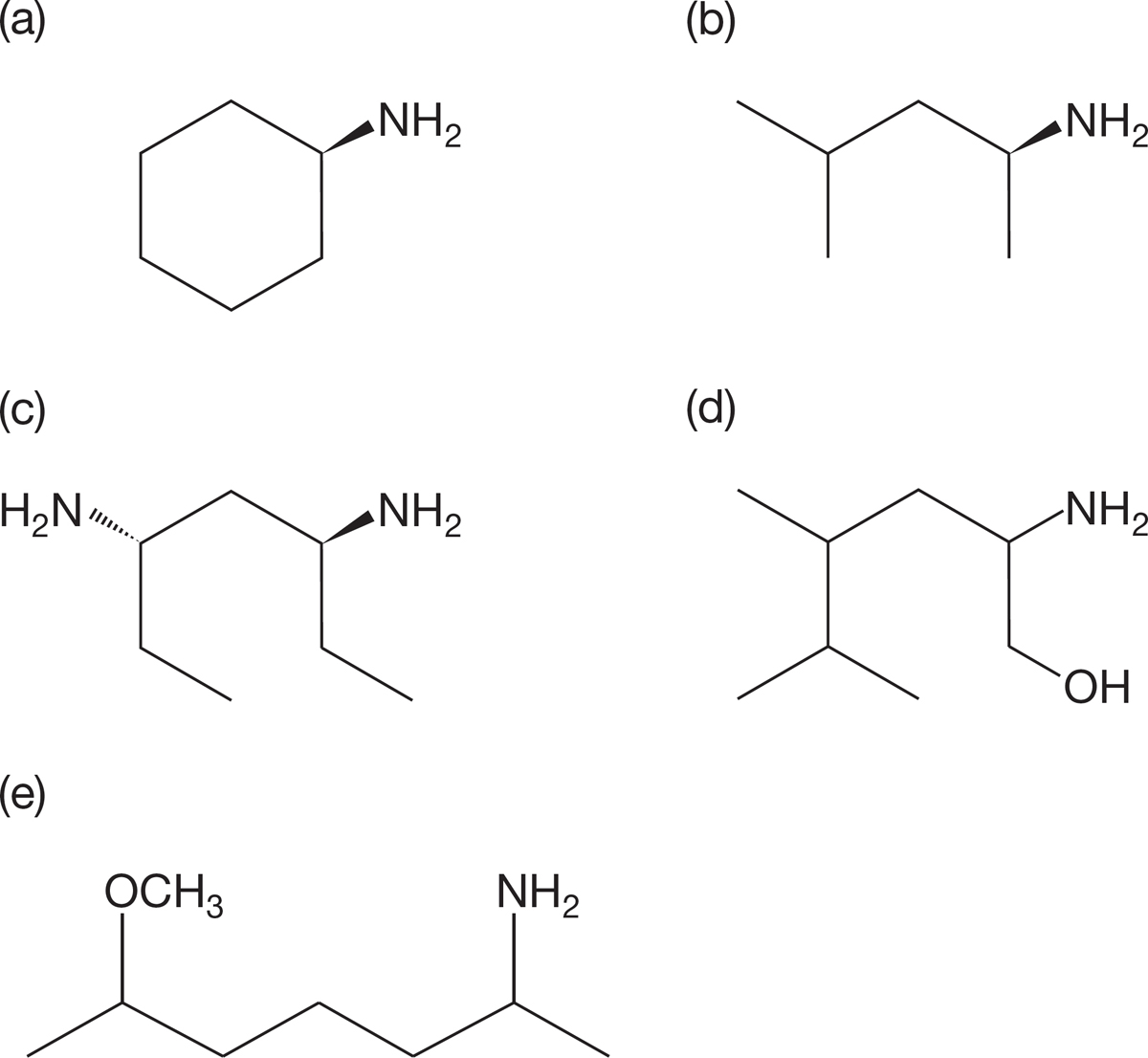
PROBLEM 6.26 Name the following compounds according to the official naming system (IUPAC):
PROBLEM 6.27 Indicate the hybridization of the carbons, nitrogens, and oxygens in each of the following compounds:
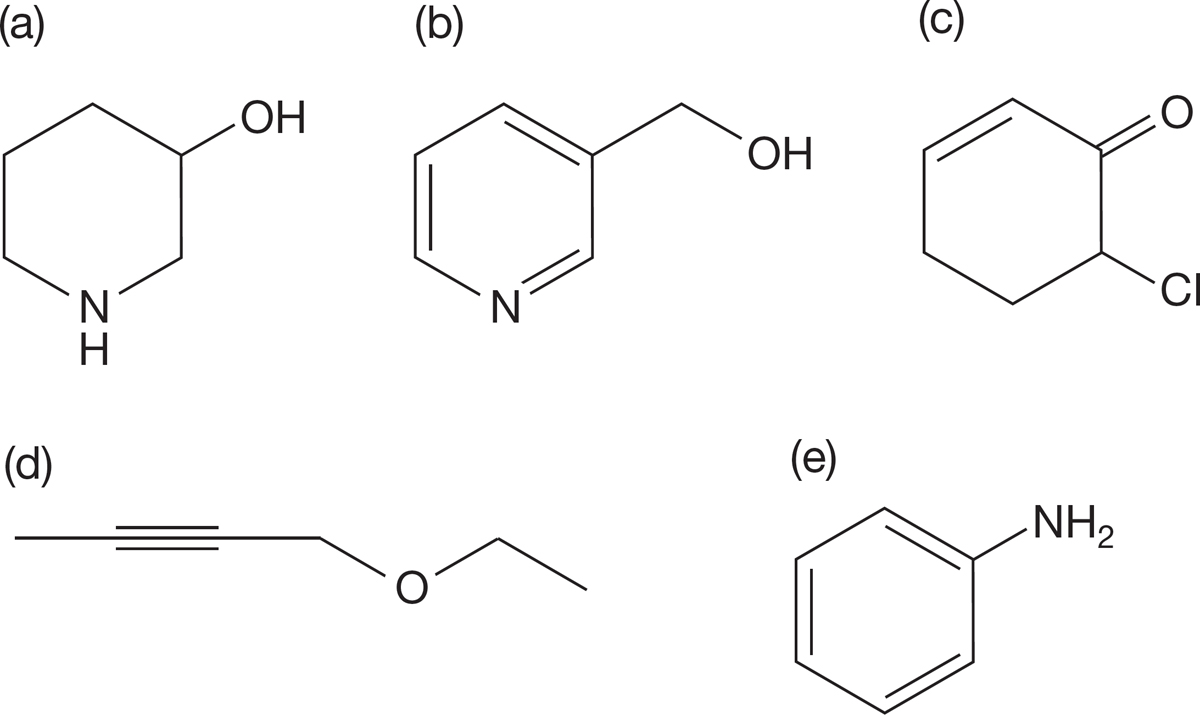
PROBLEM 6.28 Which of the molecules in Problem 6.27 are able to hydrogen bond with water? Which are able to hydrogen bond with another molecule of the same structure (i.e., with themselves)?
PROBLEM 6.29 In the following molecules, there exists the possibility of forming more than one alkoxide or thiolate when treated with base. In each case, explain which will be formed preferentially, and why.
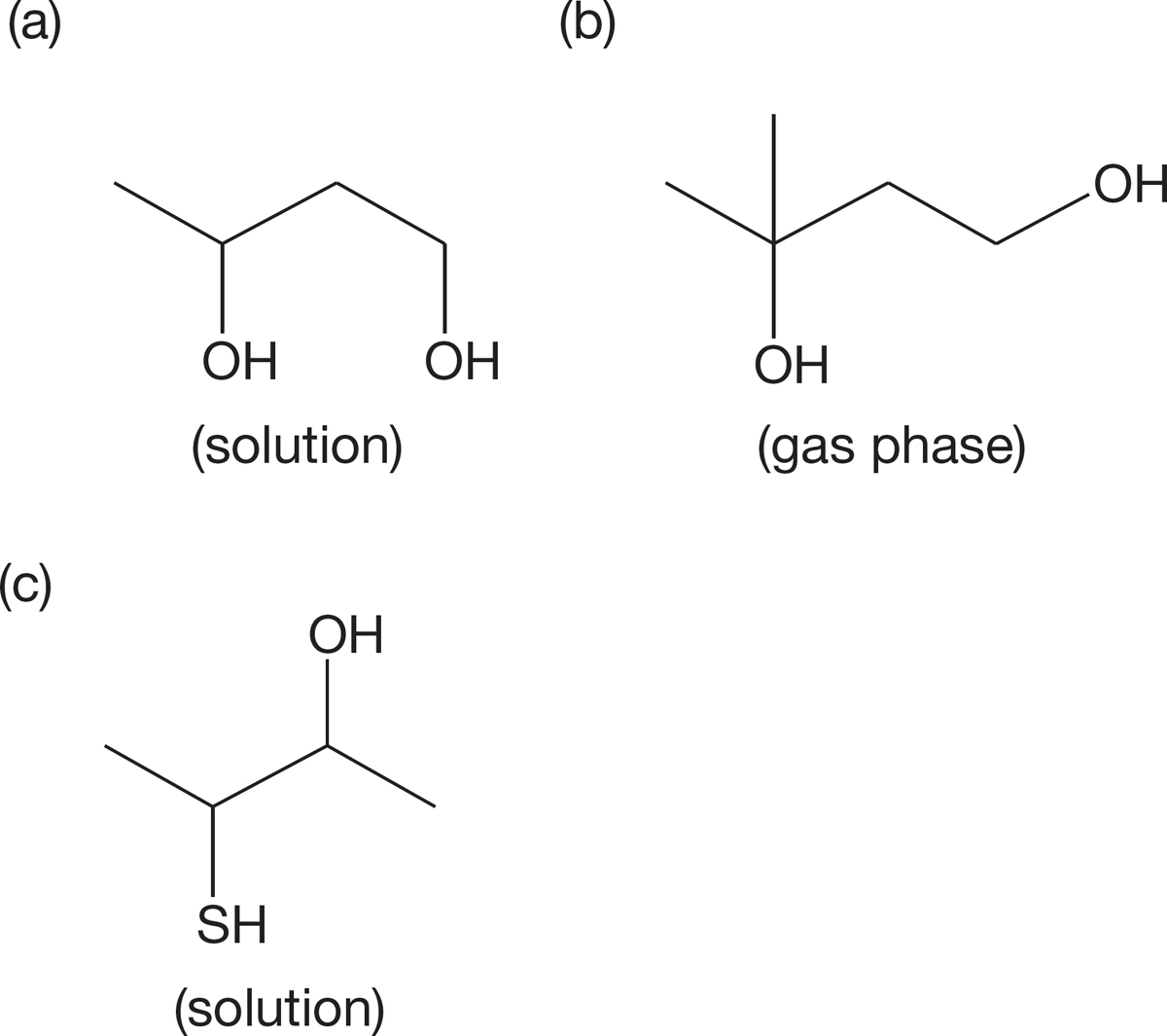
PROBLEM 6.30 Predict the order of acidities of the following three alcohols:
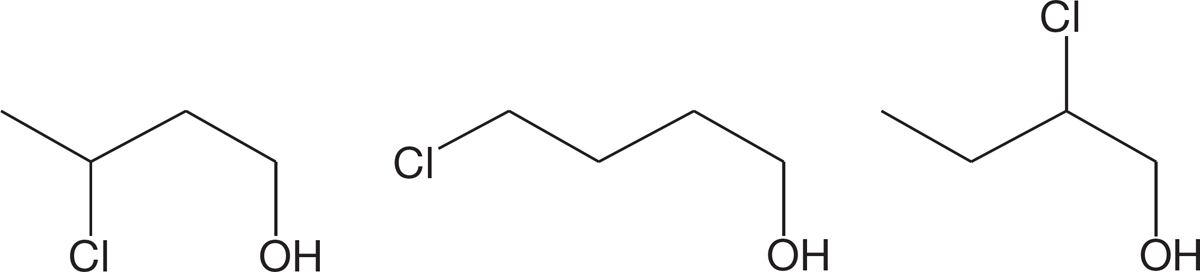
PROBLEM 6.31 Write 10 isomers of the alcohols of the formula C6H14O. Identify each of your structures as a primary, secondary, or tertiary alcohol.
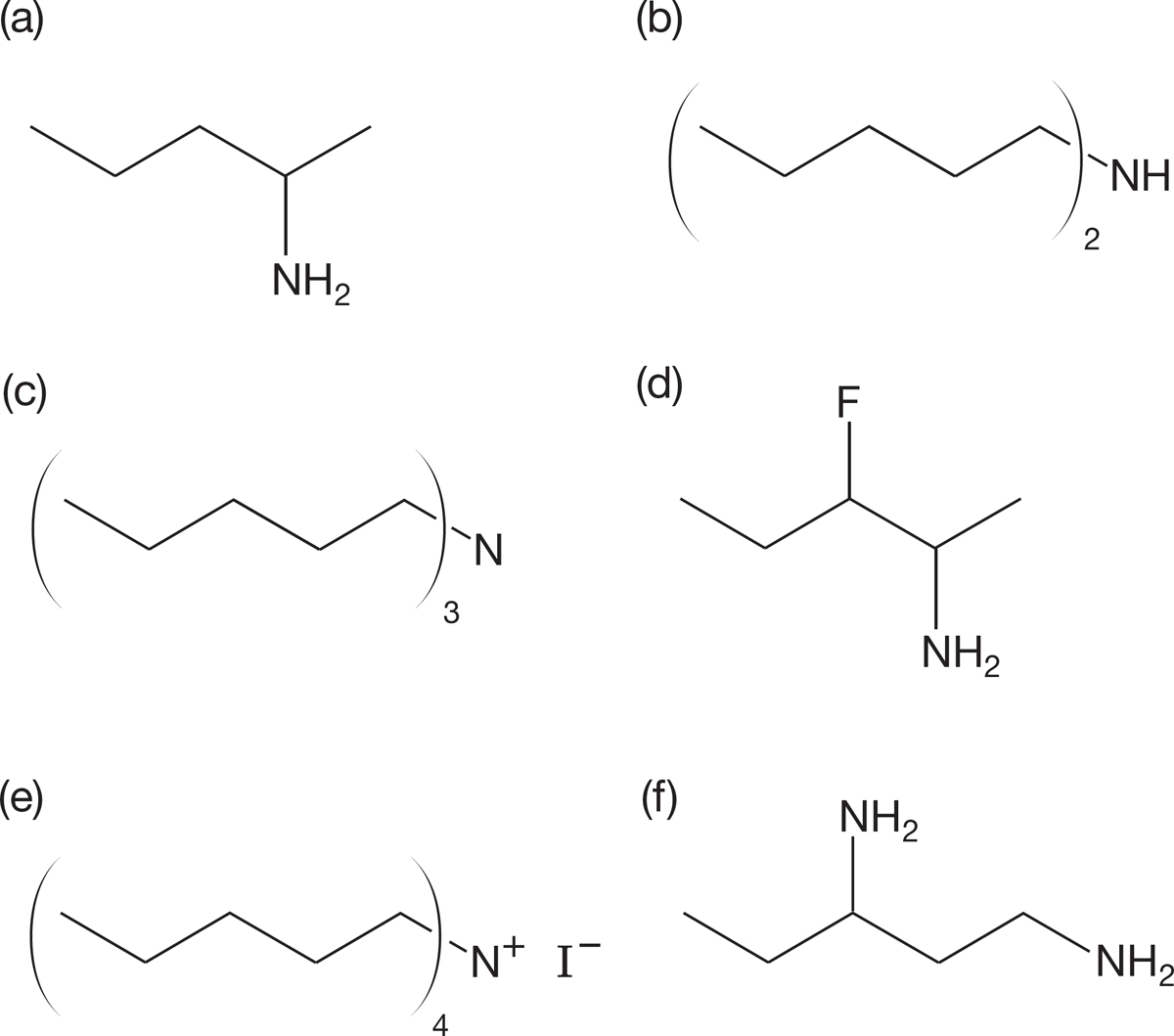
PROBLEM 6.32 Name the following compounds (in more than one way, if possible). Identify each as a primary, secondary, or tertiary amine or as a quaternary ammonium ion.
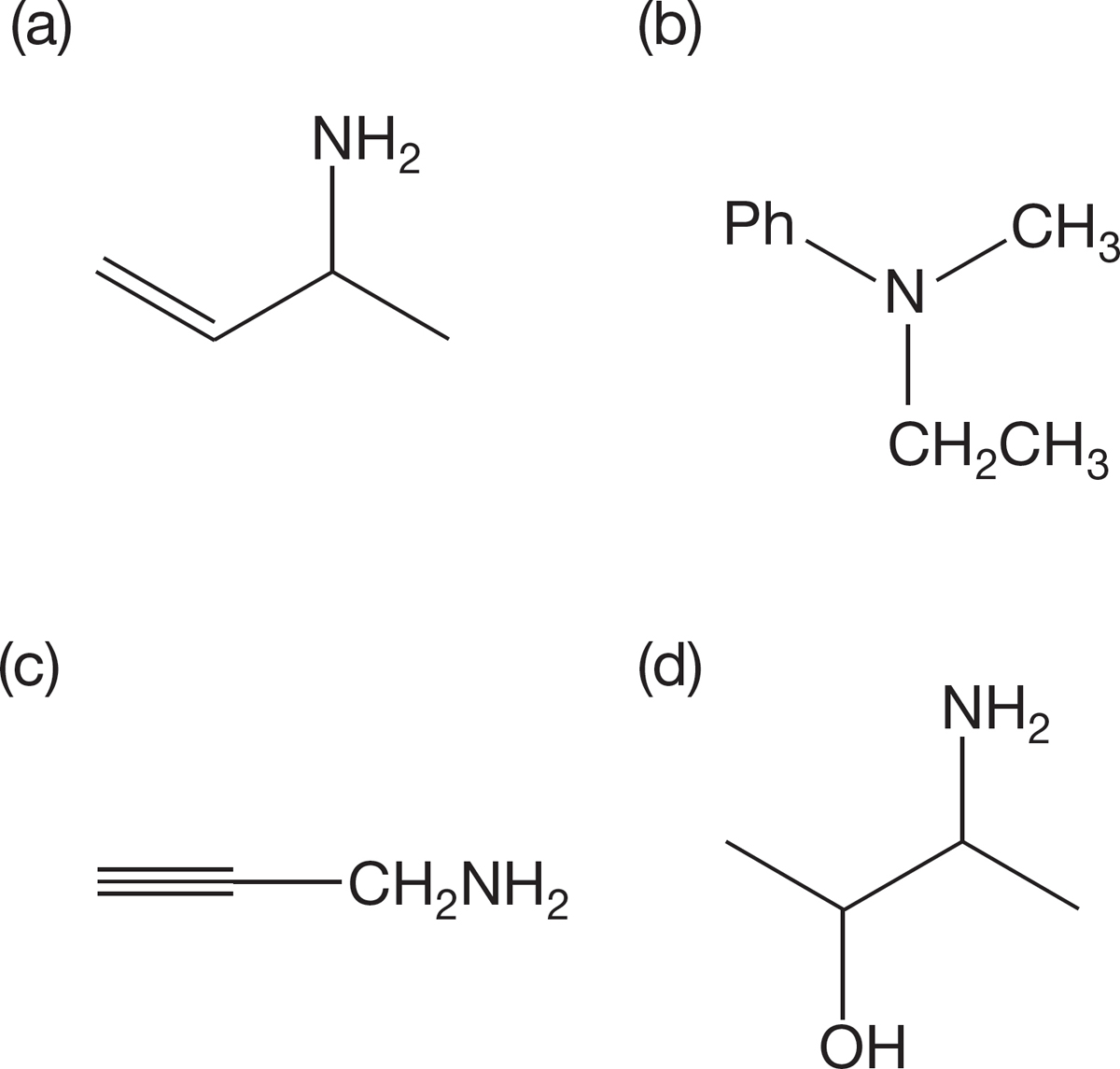
PROBLEM 6.33 Name the following compounds (in more than one way, if possible). Identify each as a primary, secondary, or tertiary amine.
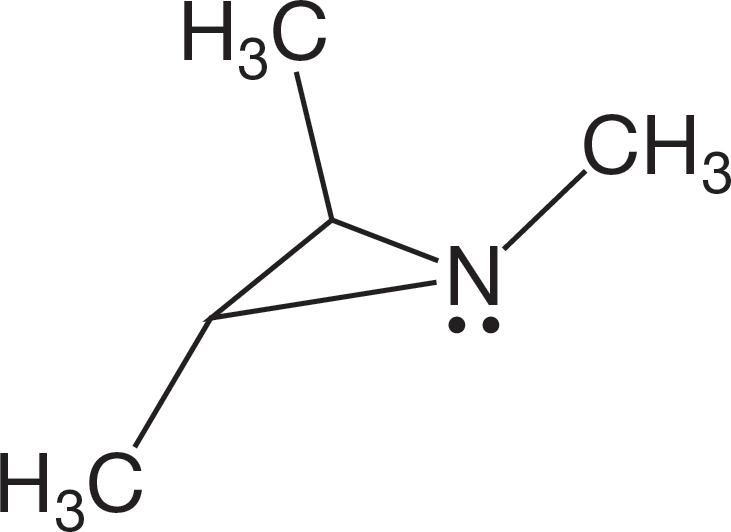
PROBLEM 6.34 Explain why the compound below shows five signals in the 13C NMR spectrum at low temperature but only three at higher temperature.
PROBLEM 6.35 Use the Internet to find the structure of lindane. Is lindane chiral? There are seven other diastereomers of lindane. Draw four of them. Circle the ones that are chiral and draw their enantiomers.
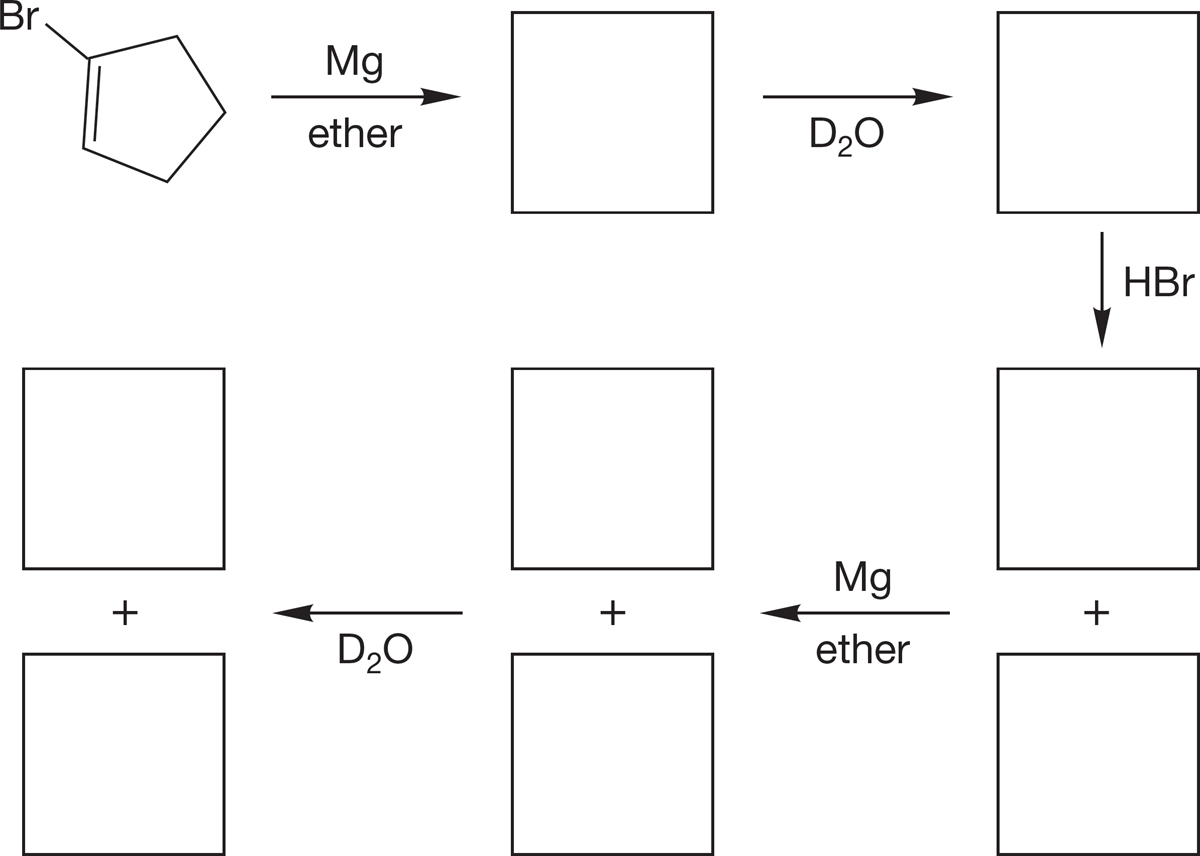
PROBLEM 6.36 Predict the products for each step in the reaction scheme.
PROBLEM 6.37 The pKb values for a series of simple amines are given below. Explain the relationship between pKb and base strength, and then rationalize the nonlinear order.
Amine |
:NH3 |
:NH2CH3 |
:NH(CH3)2 |
:N(CH3)3 |
pKb |
4.76 |
3.37 |
3.22 |
4.20 |
Use Organic Reaction Animations (ORA) to answer the following questions:
PROBLEM 6.38 Select the animation of the reaction titled “Hofmann elimination.” Observe the reagents that are involved. What type of amine is required for the reaction? Is it primary, secondary, or tertiary?
PROBLEM 6.39 How would a polar solvent impact the Hofmann elimination reaction? Would it slow the reaction down or make the reaction faster? Why? Use an energy diagram to help explain your answer.
PROBLEM 6.40 There are three products formed in the Hofmann elimination reaction. They can be seen moving off the screen at the end at the reaction. Stop the animation while all three are still on the screen. You should be able to see water, an alkene, and an amine. Which do you suppose is the best nucleophile? Select the HOMO representation for the animation. Remember that the HOMO represents the most available electrons; in other words, the most nucleophilic electrons. On the basis of the calculated HOMO, which of the three products is the most nucleophilic?
PROBLEM 6.41 Select the animation titled “Carbocation rearrangement: E1.” There are several intermediates in this reaction. Which one is the most stable? Which one is the least stable? What process is occurring in the first step of the reaction? Use Table 6.6 (p. 246) to explain why the first step occurs.
If your instructor assigns problems in  , log in at smartwork.wwnorton.com.
, log in at smartwork.wwnorton.com.

 CH
CH CH2
CH2