6.3 Structure of Substituted Alkanes
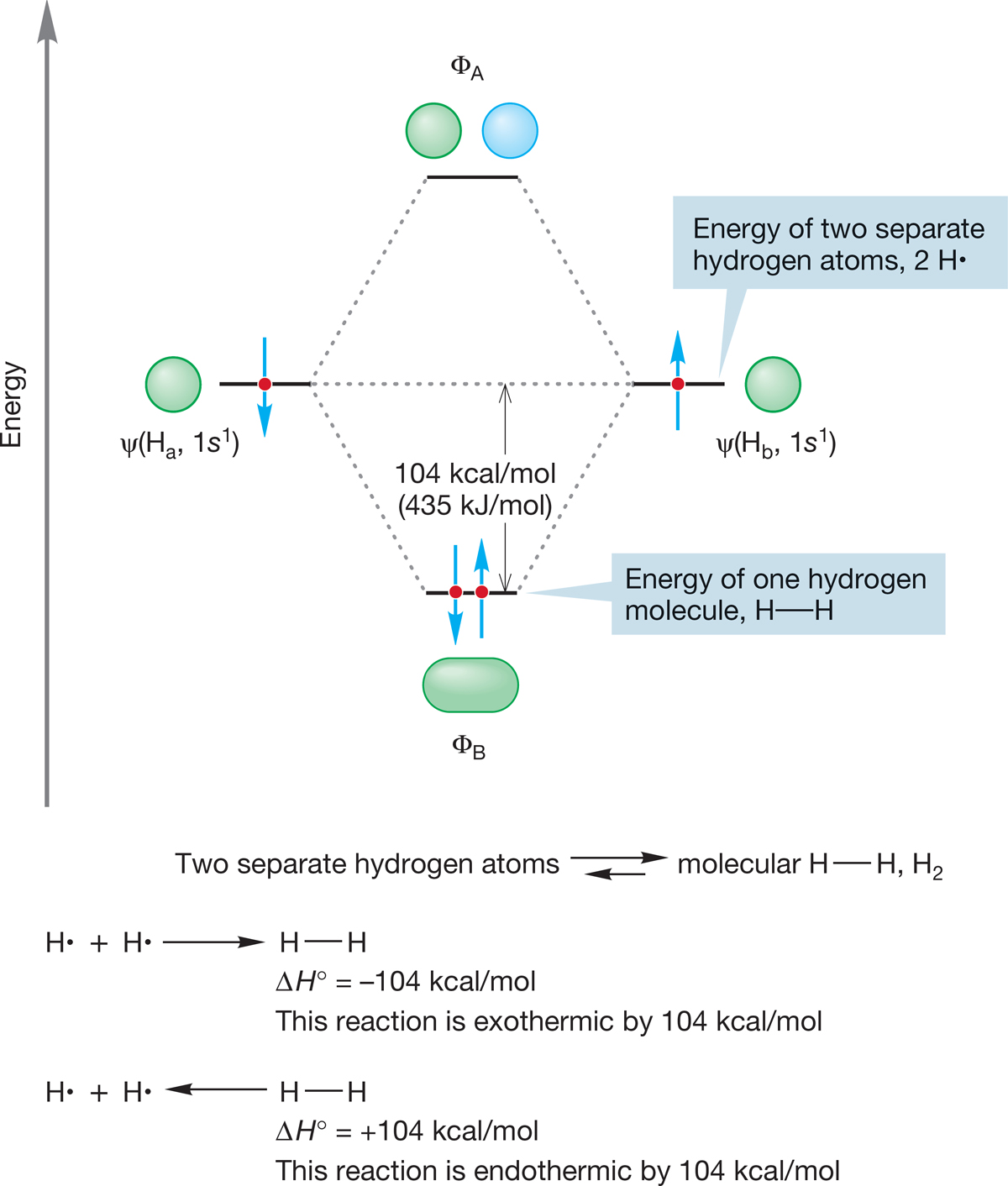
6.3a Alkyl Halides Simple alkyl halides are nearly tetrahedral, and therefore the carbon to which the halogen is attached is approximately sp3 hybridized. The bond to halogen involves an approximately sp3 carbon orbital overlapping with an orbital on the halogen for which the principal quantum number varies from n = 2 for fluorine to n = 5 for iodine. The C―X bond lengths increase and the bond strengths decrease as we read down the periodic table. Some typical values for methyl halides are shown in Figure 6.16, which gives both C―X bond lengths (in angstrom units) and C―X bond strengths (in kilocalories per mole and kilojoules per mole). Remember: These values refer to homolytic bond breaking (formation of two neutral radicals; see Fig. 1.44).
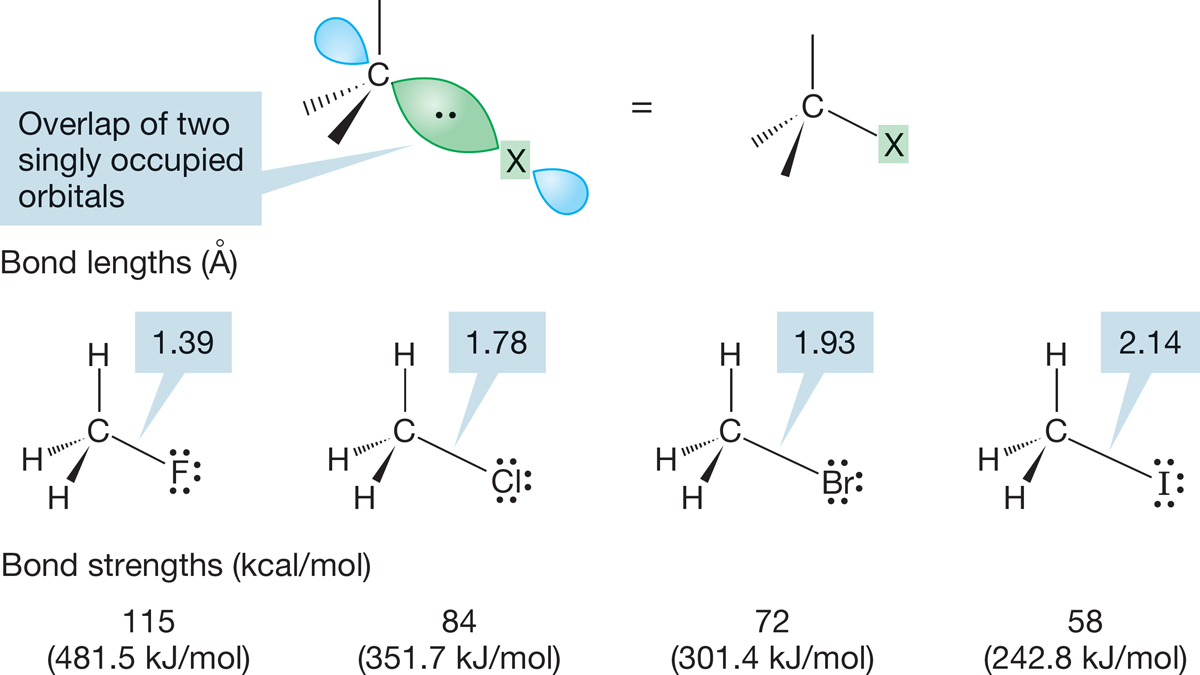
FIGURE 6.16 The C―X bond of an alkyl halide is formed by the overlap of a singly occupied carbon sp3 orbital with a singly occupied halogen orbital.
6.3b Alcohols Alcohols are derivatives of water, and it is no surprise to see that they rather closely resemble water structurally. The bond angle is expanded a bit from 104.5° in H―O―H to approximately 109° in simple alcohols R―O―H.
The oxygen–hydrogen bond length is very little changed from that of water, but the carbon–oxygen bond is a bit shorter and stronger than the carbon–carbon bond in ethane (Fig. 6.17).
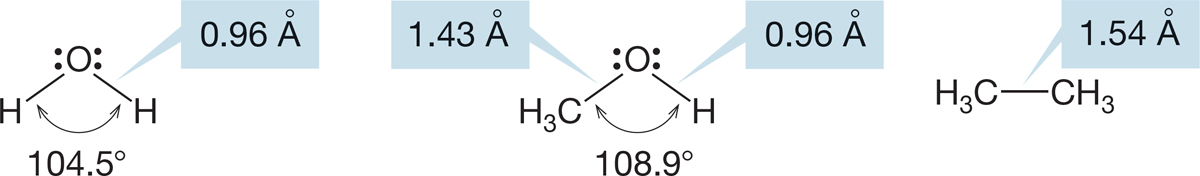
FIGURE 6.17 Bond lengths and angles for water, methyl alcohol, and ethane.
PROBLEM 6.5 What is the approximate hybridization of the oxygen atom in methyl alcohol (H3C―O―H angle = 108.9°). How do you know?
The orbital interaction diagram of Figure 6.18 shows the construction of the carbon–oxygen bond, as well as typical bond energies. Notice that an electron in an orbital near the very electronegative oxygen is more stable than it would be in an orbital near carbon.
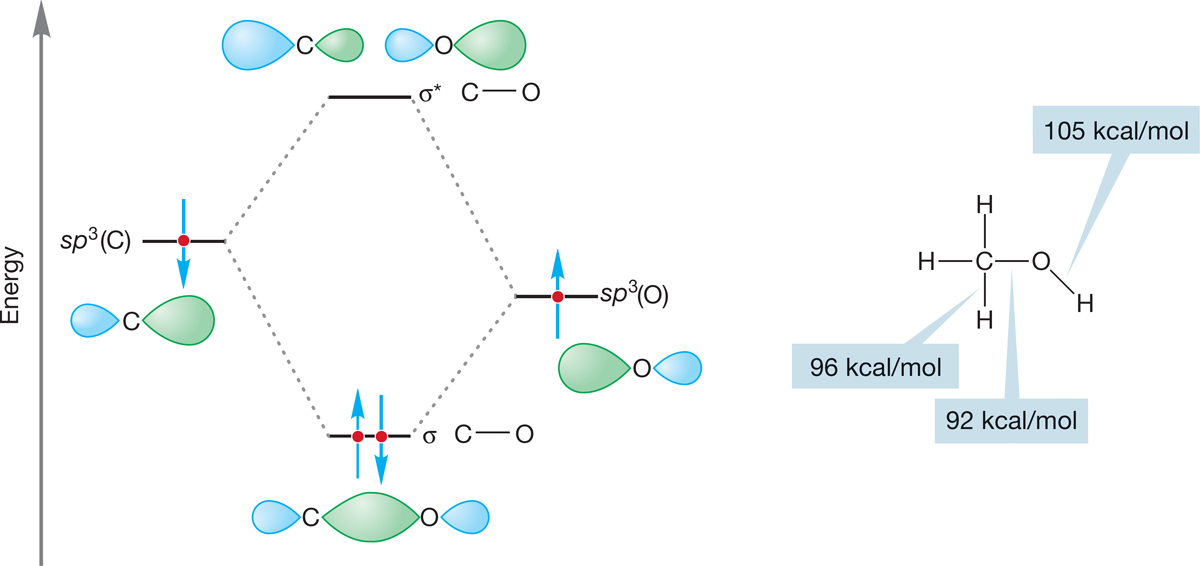
FIGURE 6.18 A graphical description of the formation of the carbon–oxygen σ bond.
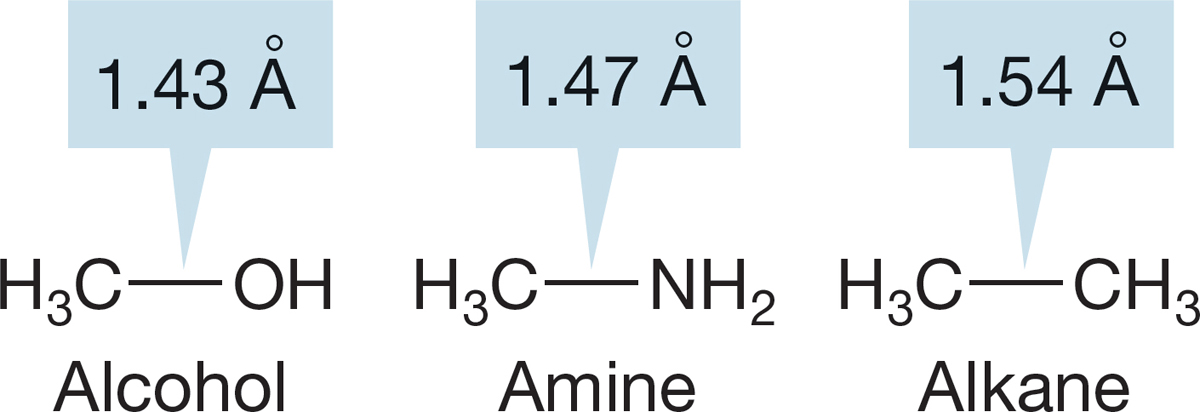
FIGURE 6.19 The carbon–nitrogen bond in amines is shorter than the carbon–carbon bond in alkanes.
6.3c Amines In simple amines, the carbon–nitrogen bond distance is a little shorter than the corresponding carbon–carbon bond distance in alkanes. This phenomenon is similar to that found in the alcohols and ethers we just discussed (Fig. 6.19).
Simple amines are hybridized approximately sp3 at nitrogen and thus are pyramidal. The bond angles are close to the tetrahedral angle of 109.5° but cannot be exactly the tetrahedral angle.
PROBLEM 6.6 Draw N,N-dimethylethanamine clearly showing the three-dimensional perspective at the nitrogen. Use the convention of solid and dashed wedges and include nitrogen’s lone pair.
PROBLEM 6.7 Why is the carbon–nitrogen bond distance in aniline slightly shorter than the carbon–nitrogen bond in methylamine?
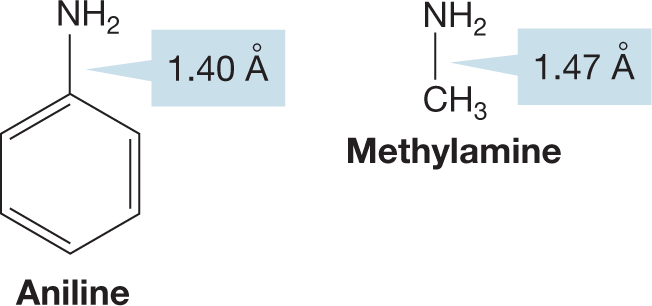
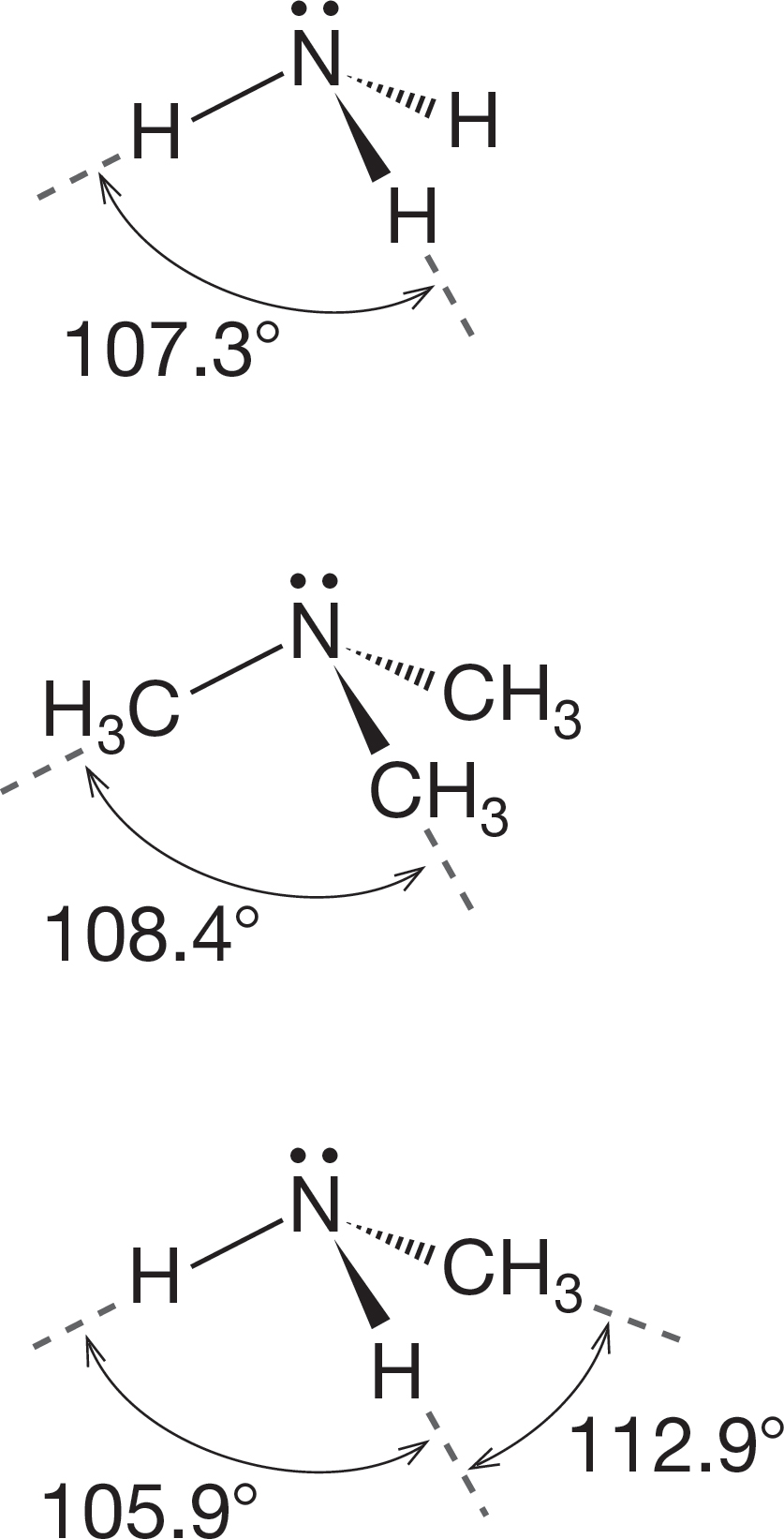
FIGURE 6.20 Some bond angles in simple amines.
For amines in which all three R groups are identical, there will be only a single R―N―R angle, but for less symmetric amines, the different R―N―R angles cannot be the same (Fig. 6.20).
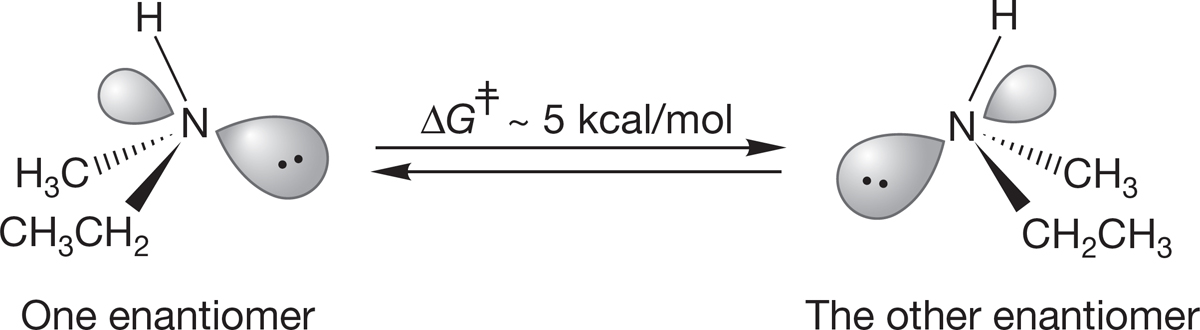
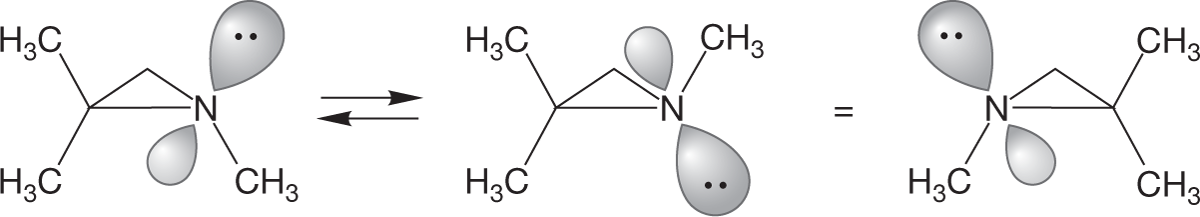
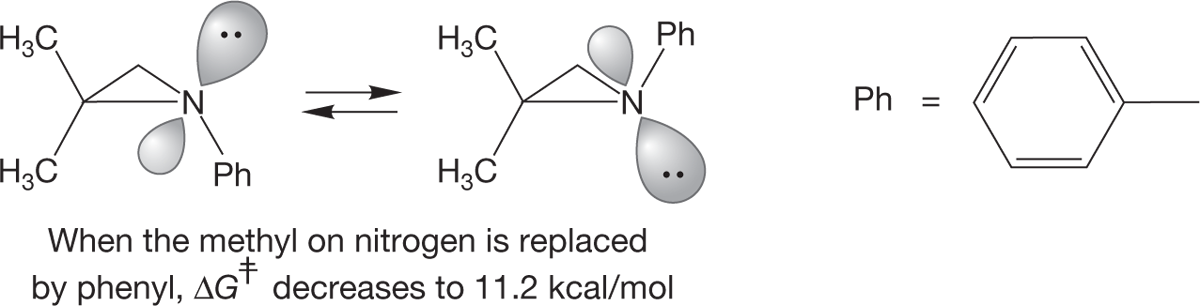
The most important structural feature of amines comes from the presence of the pair of nonbonding electrons acting as the fourth substituent on the pyramid. In alkanes, where there are four substituents, there is nothing more to consider once we describe the pyramid. In amines there is, because the pyramid can undergo an “umbrella flip,” amine inversion, forming a new, mirror-image pyramid (Fig. 6.21). Because the nitrogen can be a stereogenic center, this inversion has an interesting outcome.
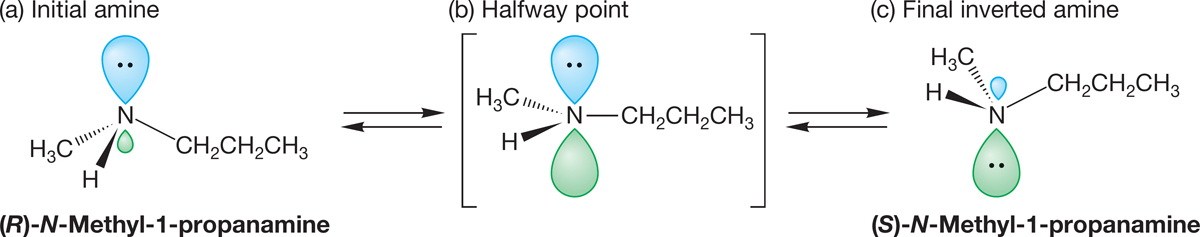
FIGURE 6.21 Amine inversion interconverts enantiomeric amines: (a) initial amine, (b) higher energy halfway point, (c) final inverted amine.