7.10 Summary
New Concepts
The most important idea in this chapter is the continuing expansion of the concept of Brønsted acidity and basicity to embrace the species known as electrophiles (Lewis acids) and nucleophiles (Lewis bases). The relatively narrow notion that acids and bases compete for a proton (Brønsted acids and bases) is extended to define all species with reactive pairs of electrons as Lewis bases and all molecules that will react with Lewis bases as Lewis acids. The key word here is reactive. All bonds are reactive under some circumstances, and therefore all molecules can act as Lewis bases at times.
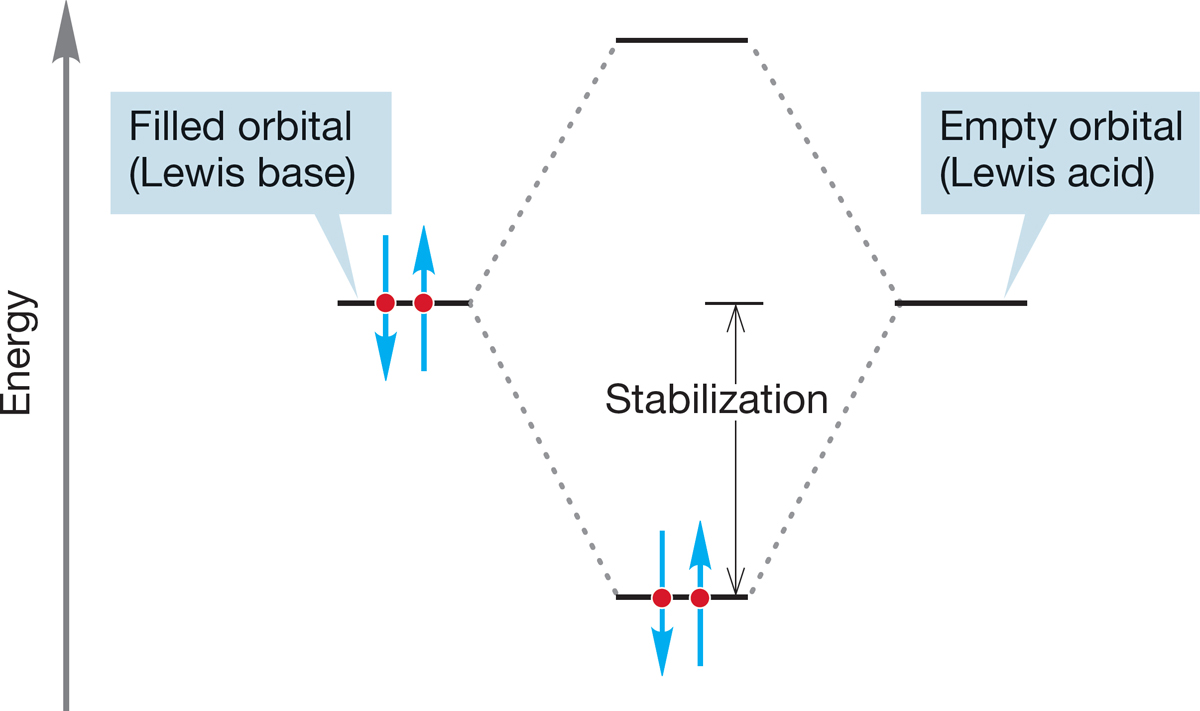
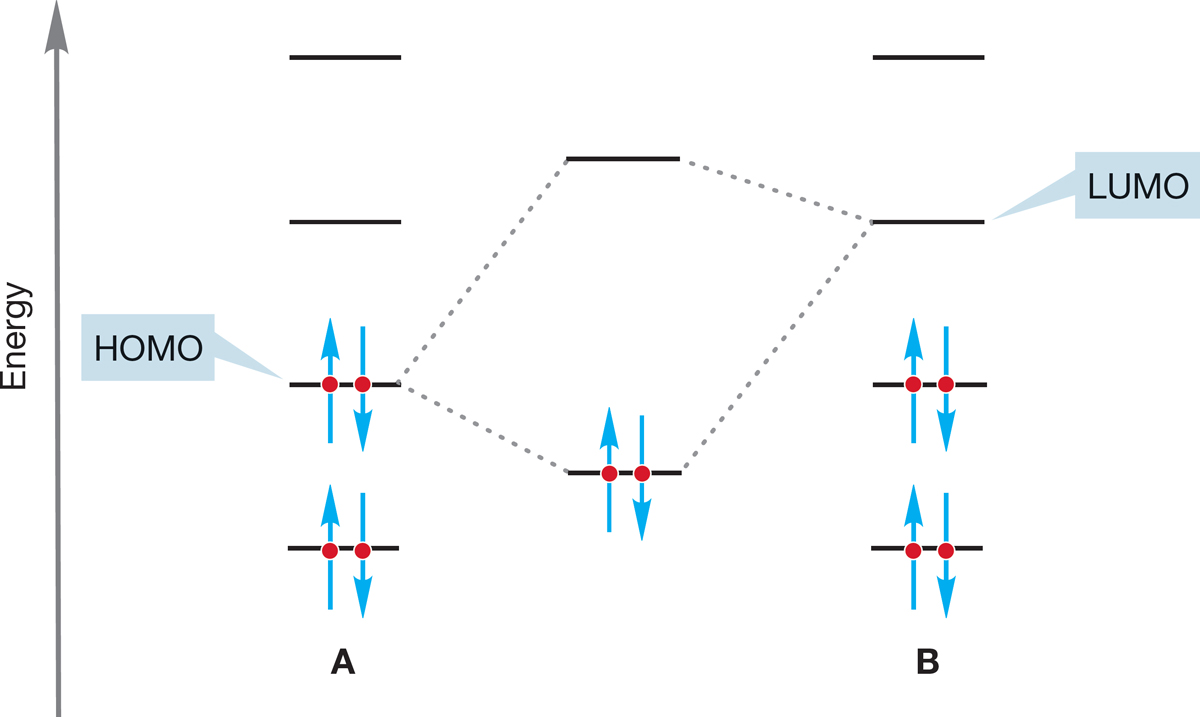
Another way to describe the reaction of Lewis bases with Lewis acids is to use the graphic device showing the stabilizing interaction of a filled orbital (Lewis base) with an empty orbital (Lewis acid) (Fig. 7.5). This idea can be generalized if you recall that the strength of orbital interaction depends on the energy difference between the orbitals involved. The closer the orbitals are to each other in energy, the stronger is their interaction and the greater is the resulting stabilization. Therefore, the strongest interactions between orbitals are likely to be between the highest occupied molecular orbital (HOMO) of one molecule (A, a Lewis base) and the lowest unoccupied molecular orbital (LUMO) of the other (B, a Lewis acid) (Fig. 7.7). This notion is used in this chapter to explain the overwhelming preference for backside attack in the SN2 reaction.
Stereochemical experiments are used here for the first time to probe the details of reaction mechanisms. The observation of inversion of configuration in the SN2 reaction shows that the incoming nucleophile must approach the leaving group from the rear, the side opposite the leaving group. The curved arrow formalism is used extensively in this chapter. This bookkeeping device is extremely useful in keeping track of the pairs of electrons involved in the making and breaking of bonds in a reaction.
The terms transition state (the structure representing the high-energy point separating starting material and products) and activation energy (the height of the transition state above the starting material) must be understood. They will be used throughout your study of organic chemistry.
Key Terms
Boltzmann distribution
endergonic
entropy change (ΔS°)
equilibrium constant (K)
exergonic
first-order reaction
Gibbs free energy change (ΔG°)
inversion of configuration
leaving group
Le Châtelier’s principle
microscopic reversibility
nucleophilicity
product-determining step
rate constant (k)
rate-determining step
reaction mechanism
retention of configuration
second-order reaction
SN1 reaction
SN2 reaction
solvolysis reaction
substitution reaction
sulfonium ion
thionyl chloride
Williamson ether synthesis
Reactions, Mechanisms, and Tools
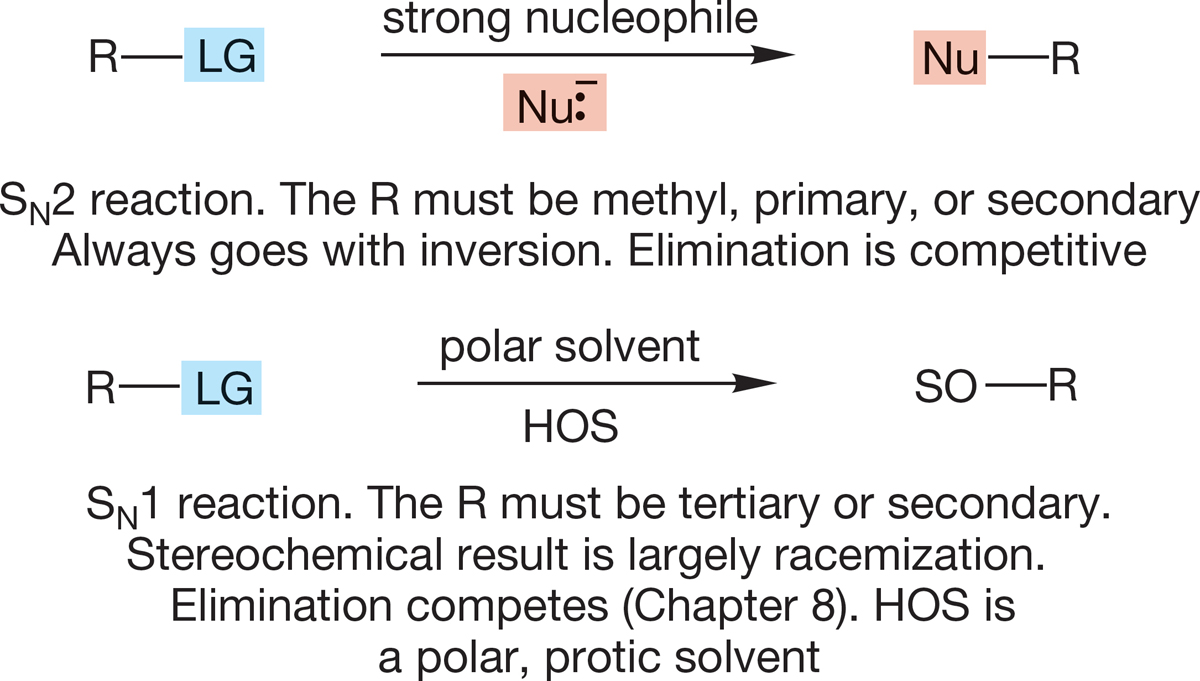
Two exceptionally important prototypal reactions are introduced in this chapter: the SN1 and SN2 substitutions. In the SN2 reaction, a nucleophile attacks the rear of the bond to the departing leaving group (backside attack). The result is inversion of configuration. In the SN1 reaction, an initial ionization to an ion pair is followed by capture of the carbocation by all available nucleophiles.
The SN2 reaction occurs only for methyl, primary, and secondary substrates, whereas the SN1 reaction occurs only for secondary and tertiary substrates. Thus, the two processes complement each other.
It is absolutely vital to have these two critically important reactions completely understood. You will encounter them often in your study of organic chemistry and they are the typical topics for standardized exams (i.e., the GRE, MCAT, DAT, and PCAT).
Syntheses
1. Substituted Alkanes
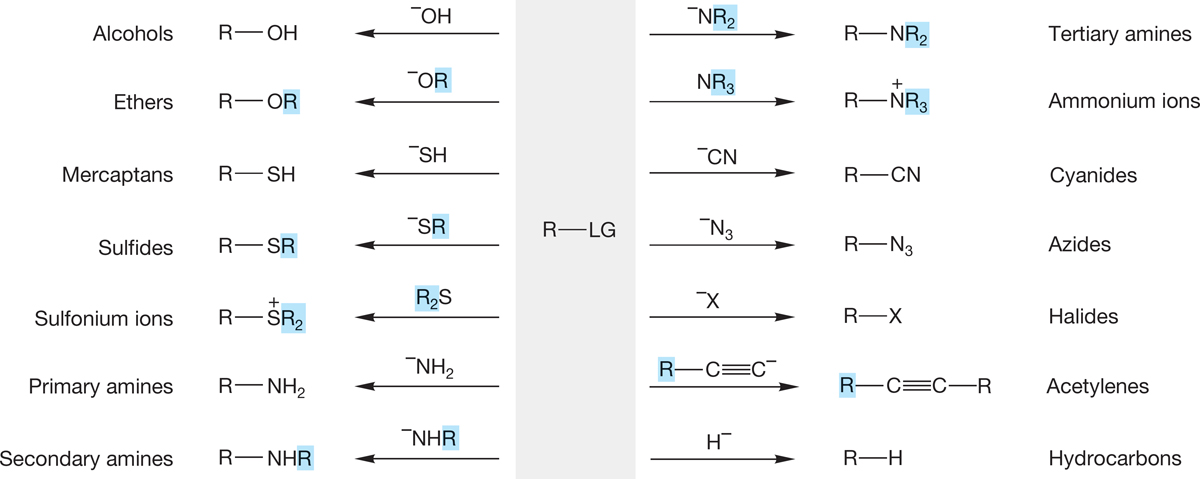
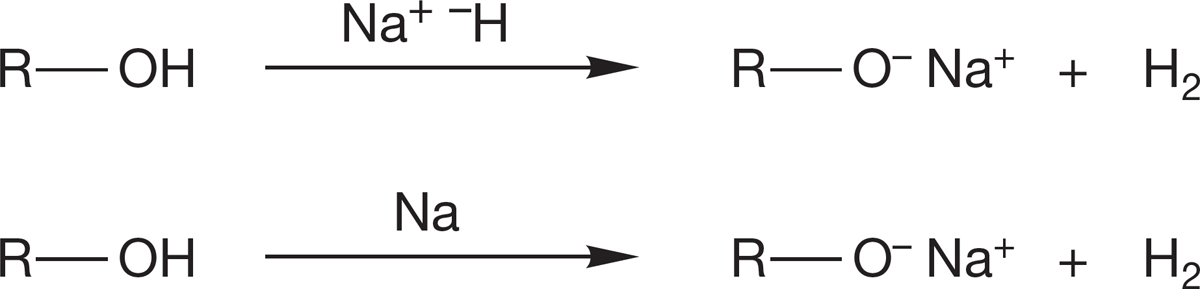
2. Alkoxides
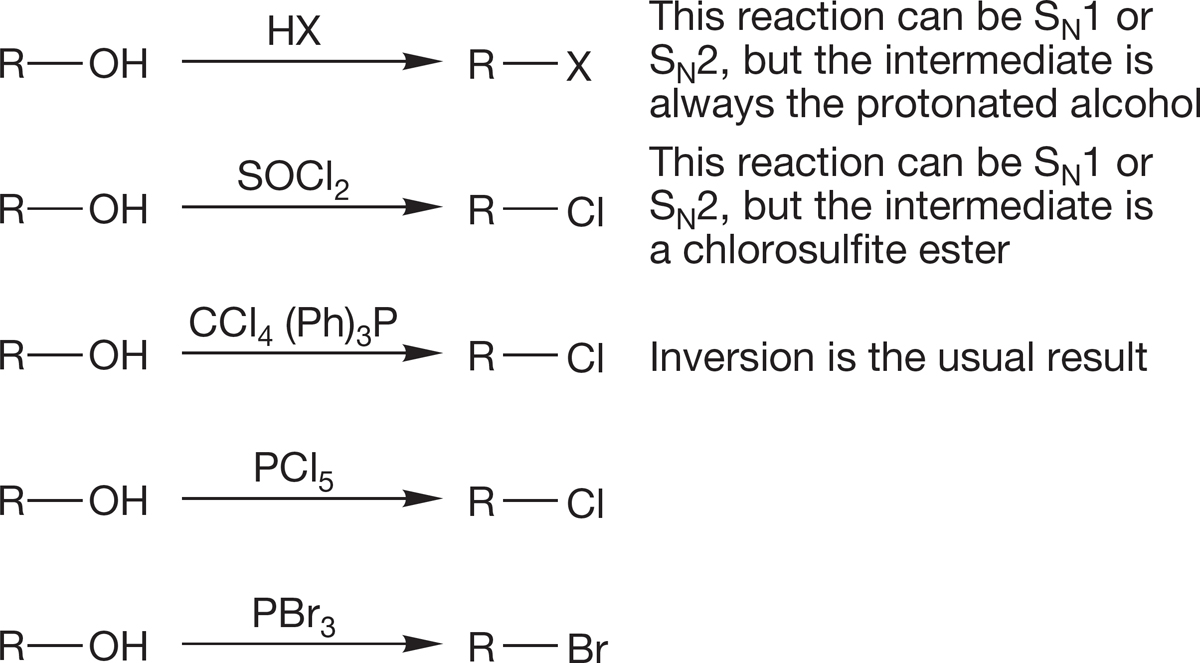
3. Alkyl Halides
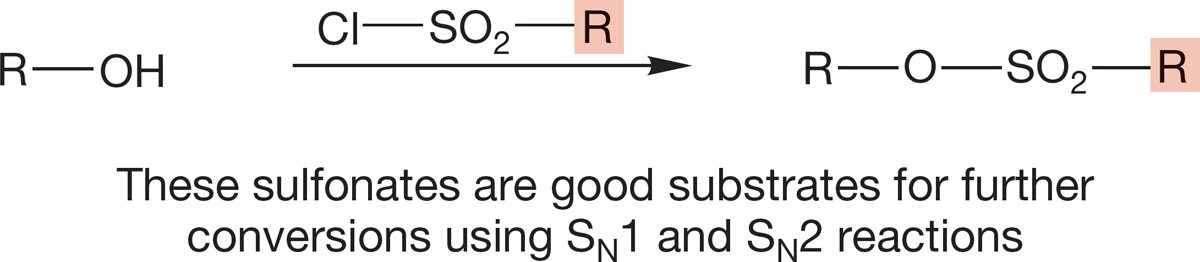
4. Alkyl Sulfonates
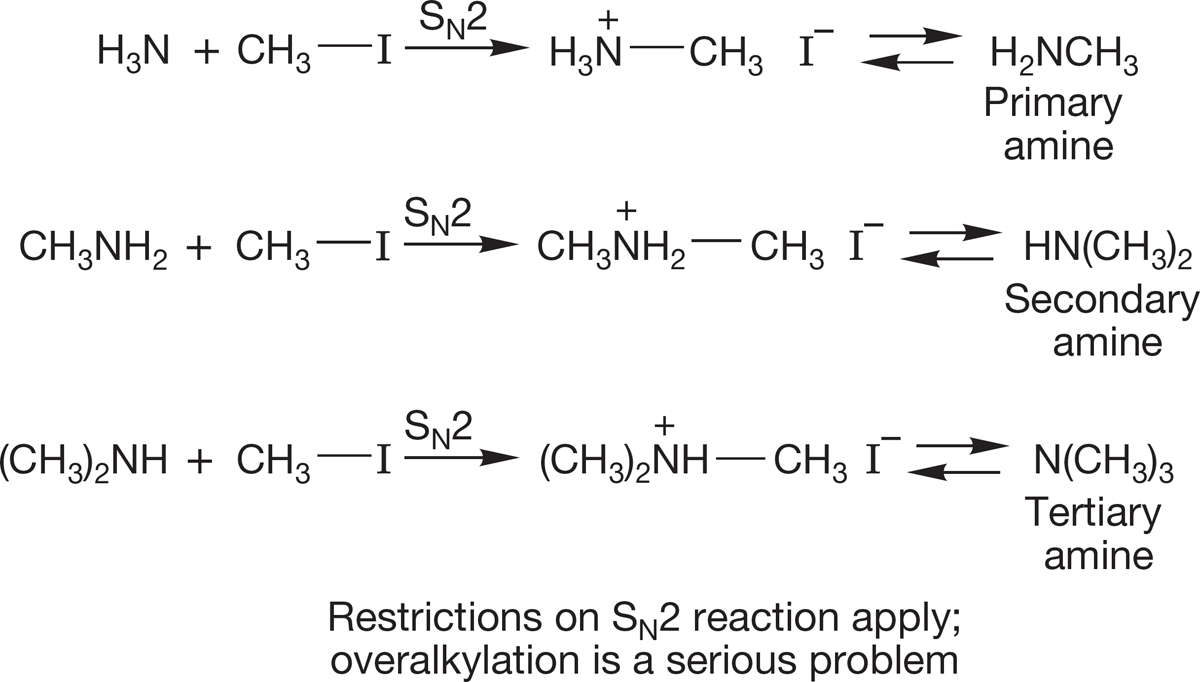
5. Amines
6. Ethers
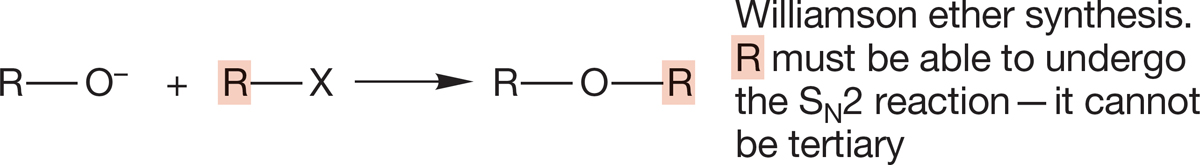
The SN1 and SN2 reactions allow the conversion of alkyl halides into a host of new structures. These reactions were summarized earlier in Figure 7.89. Remember that the SN2 reaction works only for primary and secondary halides, and the SN1 reaction works only for tertiary and secondary substrates.
Alcohols can also be used as starting materials in the SN1 and SN2 reactions, but the OH group is a very poor leaving group and must be transformed either by protonation into water or by other reactions into some other good leaving group.
Common Errors
Students in science classes often expect certainty. They have not yet learned to love the splendid messiness of organic chemistry. For example, we can ask the question: What happens if we treat A with B? The answer is very often “All sorts of things.” In this chapter, we meet two basic building blocks: the SN1 and SN2 reactions, which sometimes compete. We shall see in the next chapter that there are other competitors as well, called the E1 and E2 eliminations. It is a sad fact that in many instances, they compete with one another. We can usually adjust reaction conditions to favor the reaction we desire, but it will be a rare joyous occasion when we achieve perfect selectivity.
There is much detail in this chapter, but that is not usually a problem for most students. Good nucleophiles can be distinguished from good bases, good leaving groups can be identified, and so on. What is hard is to learn to adjust reaction conditions (reagents, temperature, and solvent) so as to achieve the desired selectivity to favor the product of just one of these four reactions. That’s hard in practice as well as in answering a question on an exam or problem set. To a certain extent, we all have to learn to live with this problem!