7.9 What Can We Do with These Reactions? How to Do Organic Synthesis
Your ability to make molecules has just increased enormously. From rather easily accessible compounds, such as the alkyl halides and inorganic materials, myriad other structural types are suddenly available. Figure 7.89 summarizes the synthetic potential of the substitution reaction. Not all reactions will work for all R―LG molecules, and as we will see in Chapter 8, other reactions often compete with substitution.
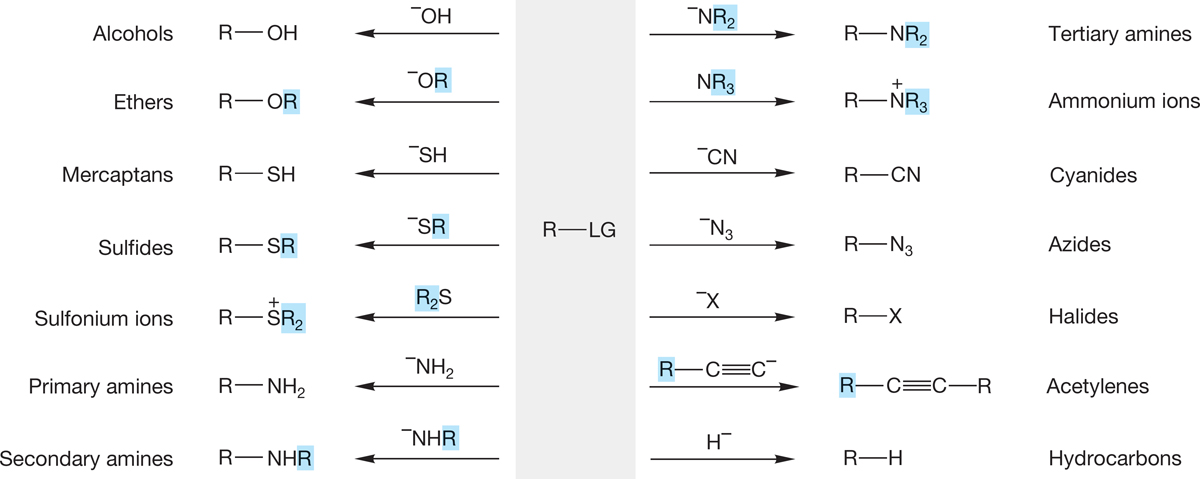
FIGURE 7.89 The synthetic potential of the substitution reaction.
Figure 7.89 adds several reactions to those we have discussed specifically. For example, it sneaks in a brand-new synthesis of substituted alkynes by using the acetylide ion as a nucleophile (usually effective only with primary R―LG compounds). We mentioned the acidity of acetylenes in Chapter 3 (p. 133) and even warned that more was coming about this reaction. Monosubstituted (terminal) alkynes are decent acids (pKa ~ 25), which means that treatment of a terminal alkyne with a strong base can result in removal of a proton and formation of the acetylide. A favorite base for doing this is the amide ion, −NH2 (Fig. 7.90). There are some other new reactions in Figure 7.89. Go over each of them carefully and be sure you see how each works.
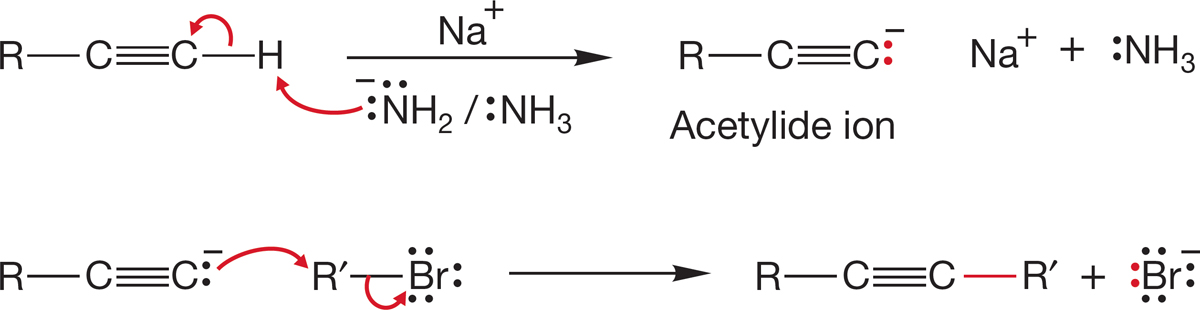
FIGURE 7.90 Formation of an acetylide from a terminal alkyne. Acetylide can be a nucleophile in an SN2 reaction.
It is relatively easy (note that we didn’t say it was easy!) to keep track of the available reactions and therefore not too difficult to think up one-step transformations. Once synthetic chemistry goes beyond one-step reactions, however, it gets much harder. Now, the intellectual challenge of synthetic chemistry increases sharply, and synthesis problems, whether in the real world or in this book, become not only more challenging but also much more fun. You can begin to see why the art of making molecules fascinates so many people. Let’s look at a few examples.

Beginning in the 1940s, the term “organic” in connection with food took on the extra meaning of “lacking use of human-made pesticides, herbicides, fertilizers, growth regulators, or genetically modified organisms.” This is clearly a misuse of the word “organic” because all food is “organic.” “Organic synthesis” deals with the formation of organic molecules, whether human-made or nature-made.
7.9a Sulfur as Nucleophile Suppose we are set the task of making a thiol from an alkyl halide. We will use the nucleophilicity of sulfur to accomplish this transformation. The mercaptide ion itself (−SH) and alkyl thiolates (−SR) are powerfully nucleophilic and can be used to great effect in the SN2 reaction. This nucleophilicity leads to the most common synthesis of thiols and sulfides. The only serious restriction is that the alkyl halide must be active in the SN2 reaction (Fig. 7.91).
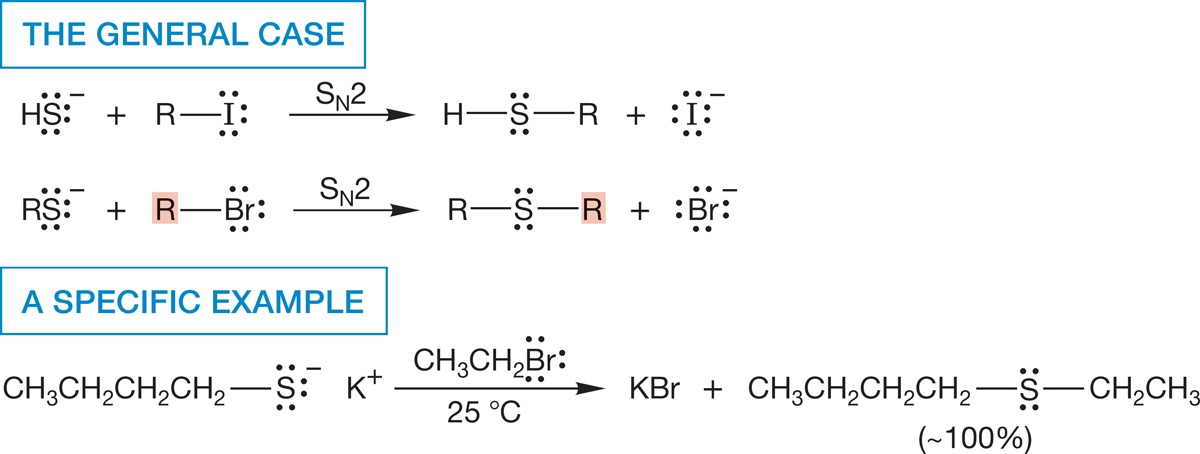
FIGURE 7.91 The SN2 reactions of mercaptide and substituted mercaptides lead to thiols and sulfides.
Sulfides have two lone pairs of electrons, and these relatives of ethers are quite nucleophilic. SN2 reactions of sulfides give sulfonium ions (Fig. 7.92).
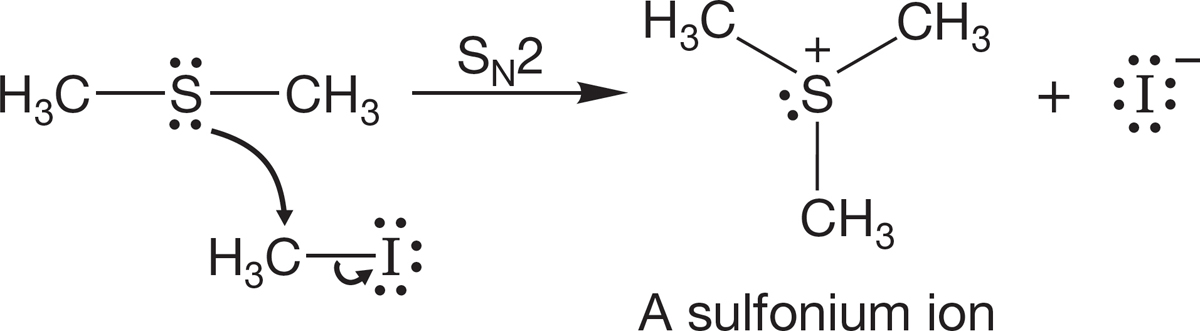
FIGURE 7.92 Sulfides can also be alkylated to give sulfonium ions.
Sulfonium ions and the related, less stable oxonium ions are used as alkylating agents. Dimethyl sulfide and dimethyl ether are excellent leaving groups and can be displaced by nucleophiles in SN2 reactions (Fig. 7.93). As we have already seen (p. 304), nature uses a variant of this reaction, displacement on the amino acid derivative S-adenosylmethionine, to do many methylation reactions.
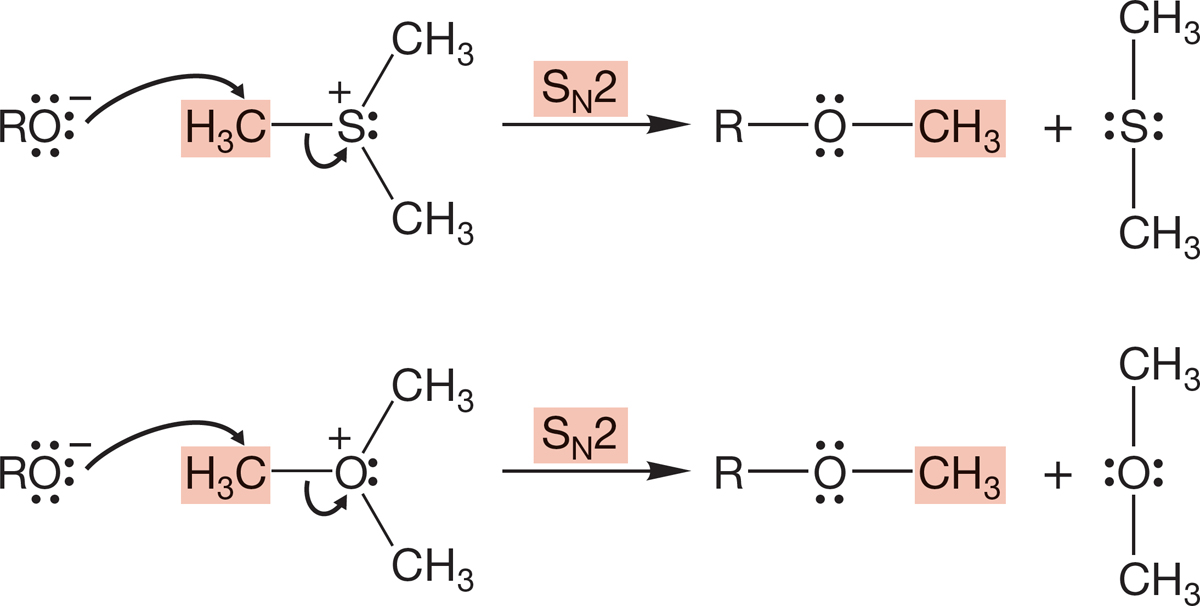
FIGURE 7.93 Alkylations by sulfonium and oxonium ions.
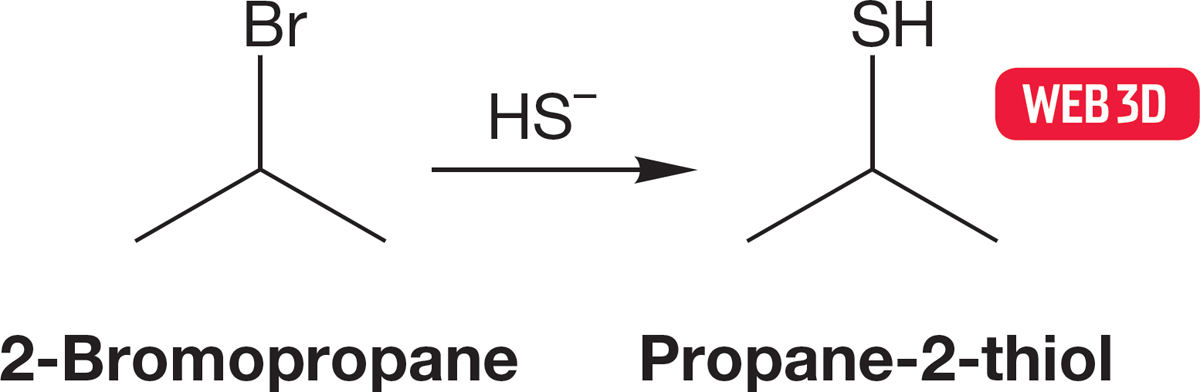
FIGURE 7.94 The conversion of 2-bromopropane into propane-2-thiol.
So, if you are set the task of synthesizing a specific thiol, say, propane-2-thiol, you have an answer right at hand: Just treat isopropyl bromide with thiolate, HS−. You might get a little elimination product as well (Chapter 8), but the reaction should work quite well. That’s an easy problem, at least if you know the reaction in Figure 7.94.
Now, however, suppose we had to make propane-2-thiol not from an alkyl halide, but from propene. We have no direct way to do this transformation, and Figure 7.89 is no help. But we do have a way to convert propene into 2-bromopropane (Chapter 3, p. 135). So, a two-step synthesis is possible (Fig. 7.95), but it is much harder to conjure up than the simple one-step method. By far, the best way to find it is to work backward from the desired product, asking yourself the question, From what immediate precursor molecule or molecules can I get the ultimate product? In the reaction we are looking at here, the answer to that question should lead you to 2-bromopropane, among others. Now ask again, From what immediate molecule or molecules can I get 2-bromopropane? The answer to that question should include propene plus HBr. Working backward in this way is the only successful route to solving problems in synthesis, and all successful students and professional chemists do it this way.
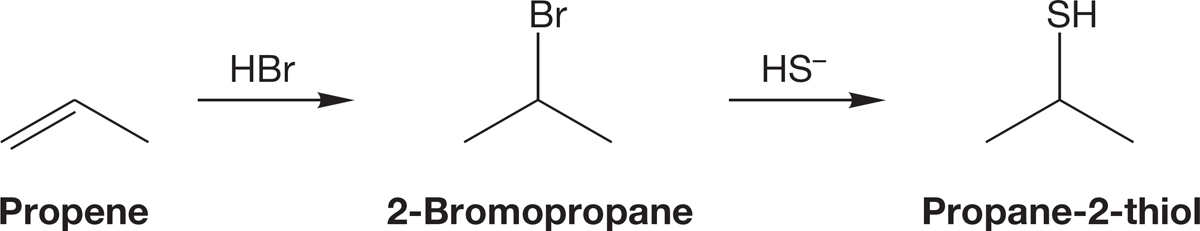
FIGURE 7.95 The two-step conversion of propene into propane-2-thiol.
Now let’s make the sequence even harder and add stereochemistry to the mix. How would you make (S)-2-butanethiol from (R)-2-butanol? Working backward tells us that we want to add thiolate (HS−) to a butyl group with a leaving group on carbon 2 and, because we want the (S) configuration in the product, that leaving group position must be the (R) configuration. You might think that we can simply protonate the (R)-2-butanol with hydrochloric acid to make it a leaving group that then will react with thiolate. But this approach will have SN1 results in addition to SN2. Another problem with this approach is that the thiolate will simply become protonated in the acidic conditions, which will make it a much less efficient nucleophile. And don’t forget, the chloride is able to compete as a nucleophile. Instead, the best way to make the alcohol into a leaving group without altering the stereochemistry is to convert it into a tosylate (p. 299). The nucleophilic thiolate can then cleanly do SN2 chemistry as shown in Figure 7.96. Working backward carefully will always lead you to a correct solution.

FIGURE 7.96 A sequence for converting (R)-2-butanol into (S)-2-butanethiol.
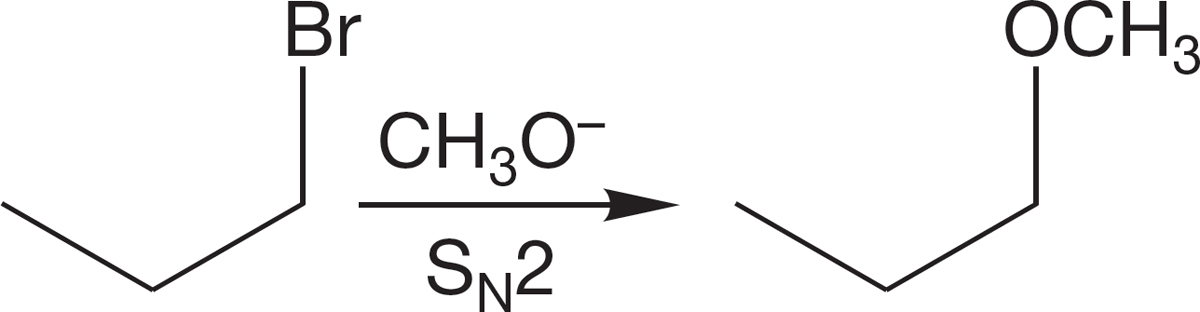
FIGURE 7.97 The Williamson ether synthesis.
7.9b Oxygen as Nucleophile: The Williamson Ether Synthesis Here’s another synthetic challenge that uses propyl bromide: Make methyl propyl ether. Given our discussion of Figure 7.94, you have almost certainly come up with the idea of displacing bromide ion from 1-bromopropane with methoxide in a straightforward SN2 sequence (Fig. 7.97). Good idea! In fact, you have just invented a good way to make ethers—well, not exactly, because A. W. Williamson (1824–1904) discovered this process, now called the Williamson ether synthesis, more than 100 years ago (Fig. 7.98). This synthesis has both limitations and opportunities, and they rather nicely illustrate some of the kinds of thinking that synthetic chemists have to go through. So let’s explore the Williamson synthesis a bit.
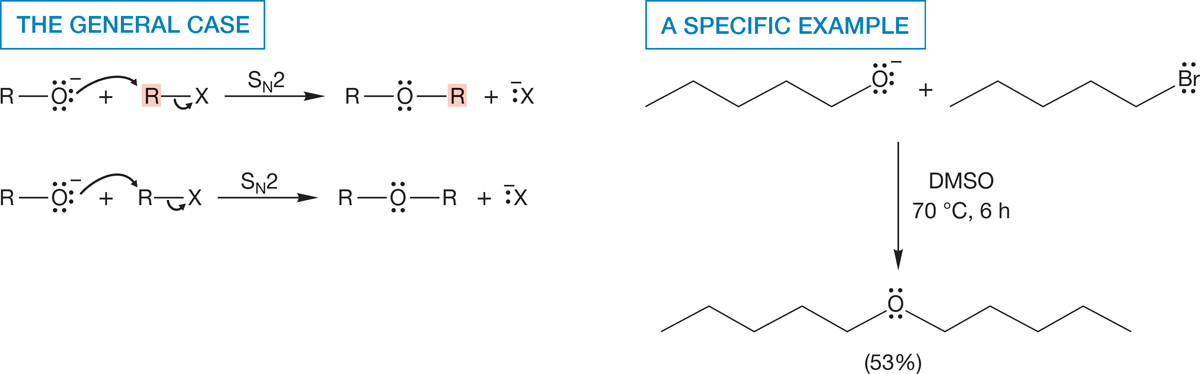
FIGURE 7.98 Alkoxides can displace halides in an SN2 reaction to make ethers. This reaction is the Williamson ether synthesis.
First of all, we need to be able to make the necessary alkoxide. As we saw in Chapter 6 (p. 247), when an alcohol is treated with a much stronger base, it can be converted entirely into the alkoxide. Note that the base used must be much stronger than the alkoxide. If that is not the case, an equilibrium mixture of the base and the alkoxide will result. A favorite reagent for formation of alkoxides is sodium hydride (Fig. 7.99). Sodium hydride has the advantage of producing hydrogen gas, which can easily be removed from the reaction mixture.
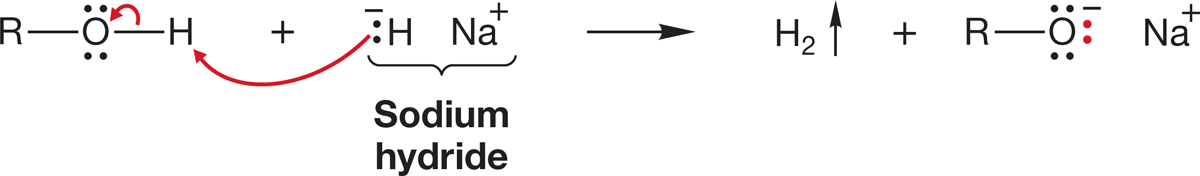
FIGURE 7.99 Sodium hydride irreversibly removes a proton to give the alkoxide, the conjugate base of the alcohol.
Just as water reacts with metallic sodium or potassium to give the corresponding metal hydroxide, alkoxides can be made through a reaction in which sodium or potassium metal is dissolved directly in the alcohol. Hydrogen is liberated in what can be a most vigorous reaction indeed. Potassium is particularly active in all cases, and the reaction of sodium metal with smaller alcohols, such as methyl and ethyl alcohol, is also rapid and exothermic (Fig. 7.100).

FIGURE 7.100 Alkoxides can be made through the reaction of metallic sodium or potassium with alcohols.
WORKED PROBLEM 7.27 Is a molar equivalent amount of sodium hydroxide an effective reagent for formation of sodium ethoxide from ethyl alcohol? Explain your answer using Table 7.6.

ANSWER The pKa values for water (15.7) and ethyl alcohol (15.9) are very close, so the equilibrium concentration of sodium ethoxide will be slightly less than that of hydroxide ion. Hydroxide ion is a slightly weaker base than ethoxide ion in solution. Thus, hydroxide will not be effective in making large amounts of ethoxide. At equilibrium, both species will be present:

Now let’s use alkoxides to make ethers—the Williamson ether synthesis. Several modifications of the original procedure have made this venerable method quite useful, although there are some restrictions. The reaction works only for alkyl halides that are active in the SN2 reaction. Therefore, tertiary halides cannot be used. They are too hindered to undergo the crucial SN2 reaction. Sometimes there is an easy way around this problem, but sometimes there isn’t. For example, tert-butyl methyl ether cannot be made from tert-butyl iodide and sodium methoxide (these conditions lead to elimination as we will see in Chapter 8), but it can be made from tert-butoxide and methyl iodide (Fig. 7.101). However, there is no way to use the Williamson ether synthesis to make di-tert-butyl ether.
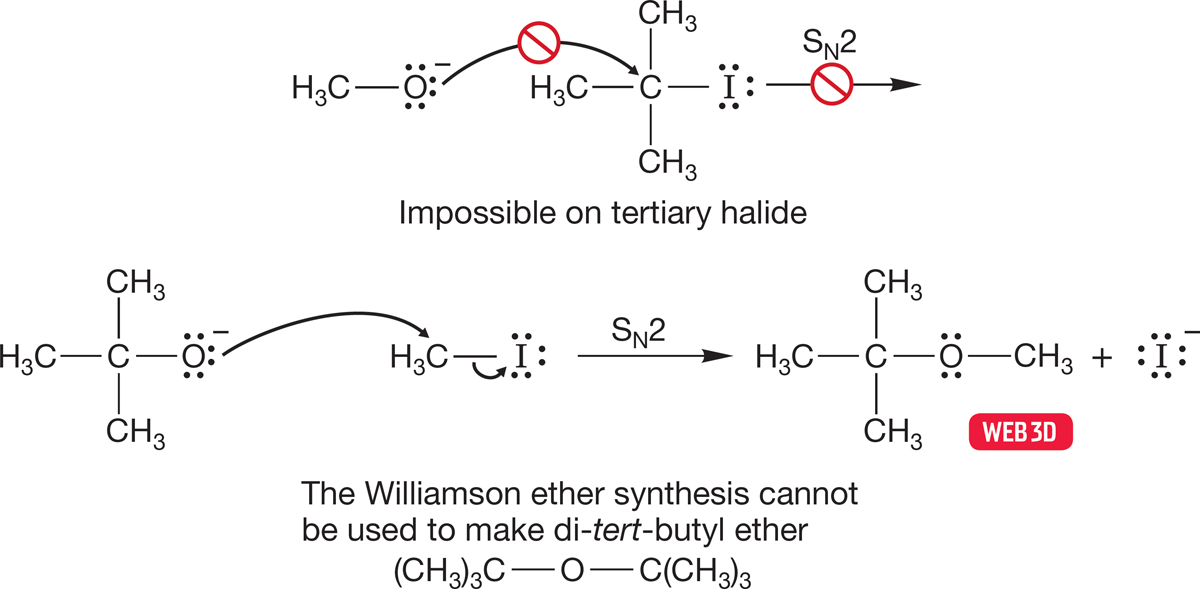
FIGURE 7.101 How to use the Williamson ether synthesis.
Even secondary halides can be problematic in the Williamson reaction. Alkoxides are strong bases (high affinity for a hydrogen 1s orbital), and elimination rather than substitution is often the major reaction when a secondary halide is used (Fig. 7.102). We will learn about this “E2” elimination reaction in Chapter 8.
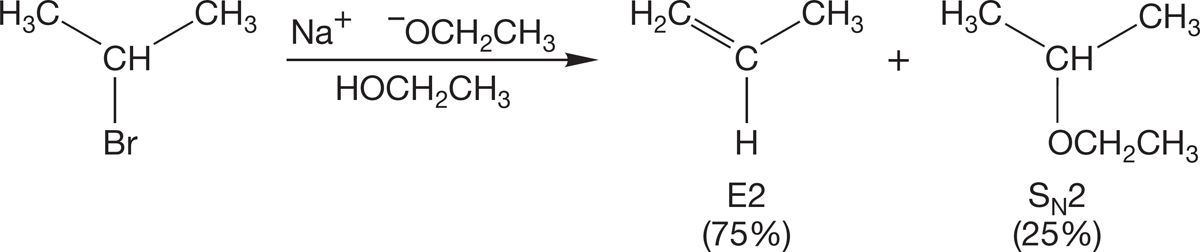
FIGURE 7.102 Secondary halides undergo substantial elimination with alkoxides.
Be alert for intramolecular, ring-forming versions of the Williamson ether synthesis. Cyclic ethers can be formed by intramolecular SN2 reactions. There is no mystery in this reaction; it is simply the acyclic process applied in an intramolecular way (Fig. 7.103).
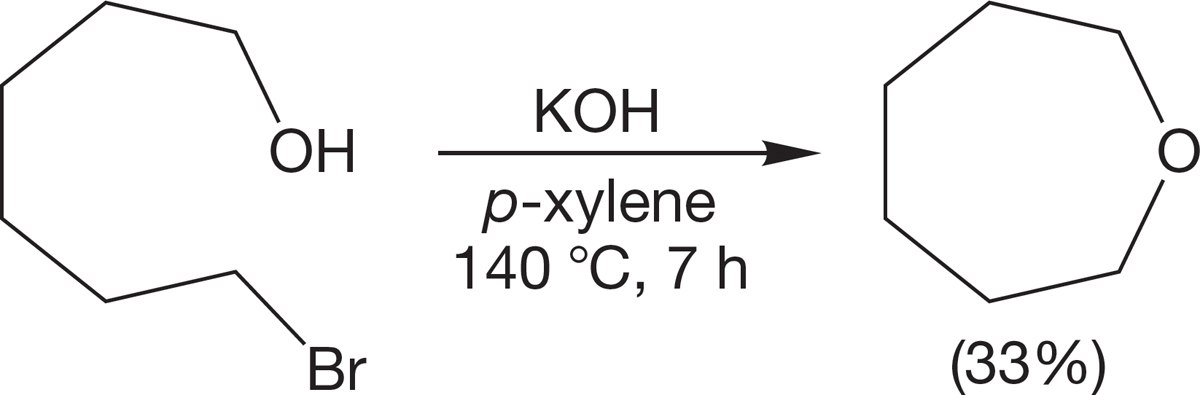
FIGURE 7.103 An intramolecular Williamson ether synthesis.
An important use of the intramolecular Williamson ether synthesis is in the making of epoxides (p. 237). A 2-halo-1-hydroxy compound (a halohydrin) is treated with base to form the haloalkoxide, which then undergoes an intramolecular backside SN2 displacement to give the three-membered ring (Fig. 7.104).
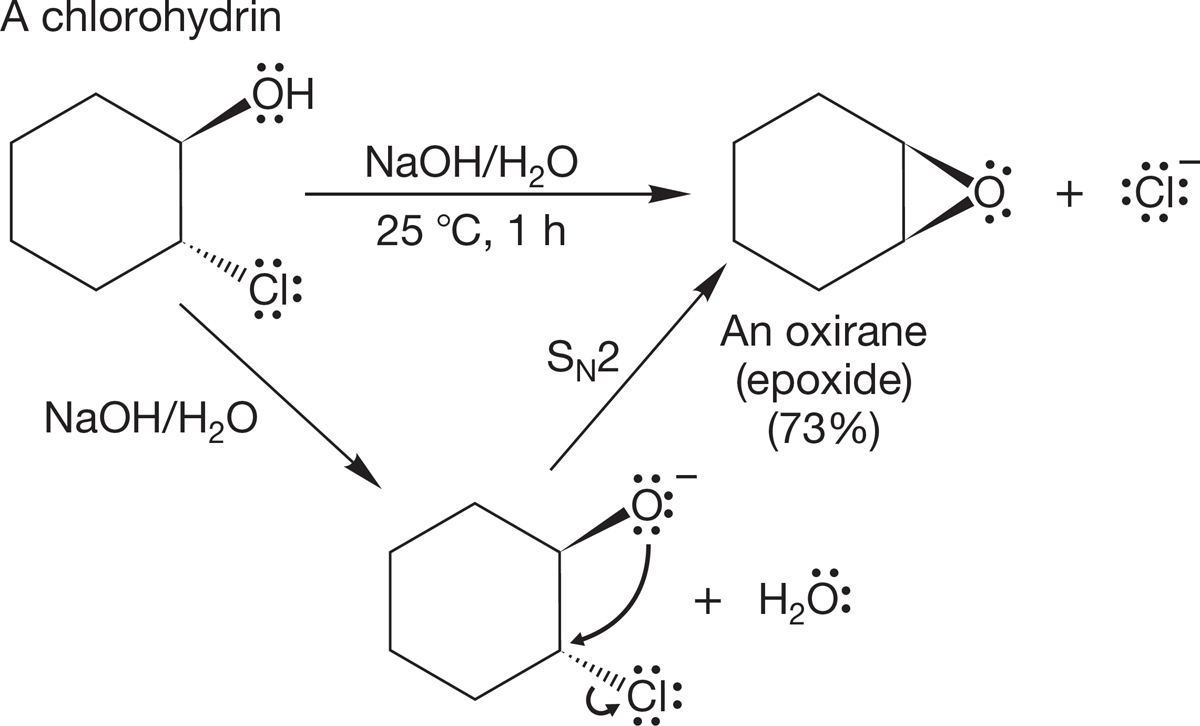
FIGURE 7.104 A common epoxide synthesis takes advantage of an intramolecular SN2 reaction.
7.9c Nitrogen as Nucleophile: Alkylation of Amines We have just examined a series of synthetically useful reactions that uses oxygen as the nucleophile. Nitrogen has a lone pair of electrons and therefore is also nucleophilic. Let’s look here at some synthetic reactions in which nitrogen is the nucleophile. Conceptually, the following reactions are rather closely related to the Williamson ether synthesis.
Amines are good nucleophiles and can displace leaving groups in the SN2 reaction. Of course, the limitations of the SN2 reaction also apply—there can be no displacements at tertiary carbons. Successive displacement reactions yield primary, secondary, and tertiary amines. The initial products are alkylammonium ions, which are subsequently deprotonated by the amine present. These SN2 reactions produce amines that are also nucleophilic. Further displacement reactions are possible, which can lead to problems. Indeed, the product amine is often a better participant in the SN2 reaction than the starting material. Therefore, it can be difficult to stop the reaction at the desired point, even if an excess of the starting amine is used to minimize overalkylation (Fig. 7.105).
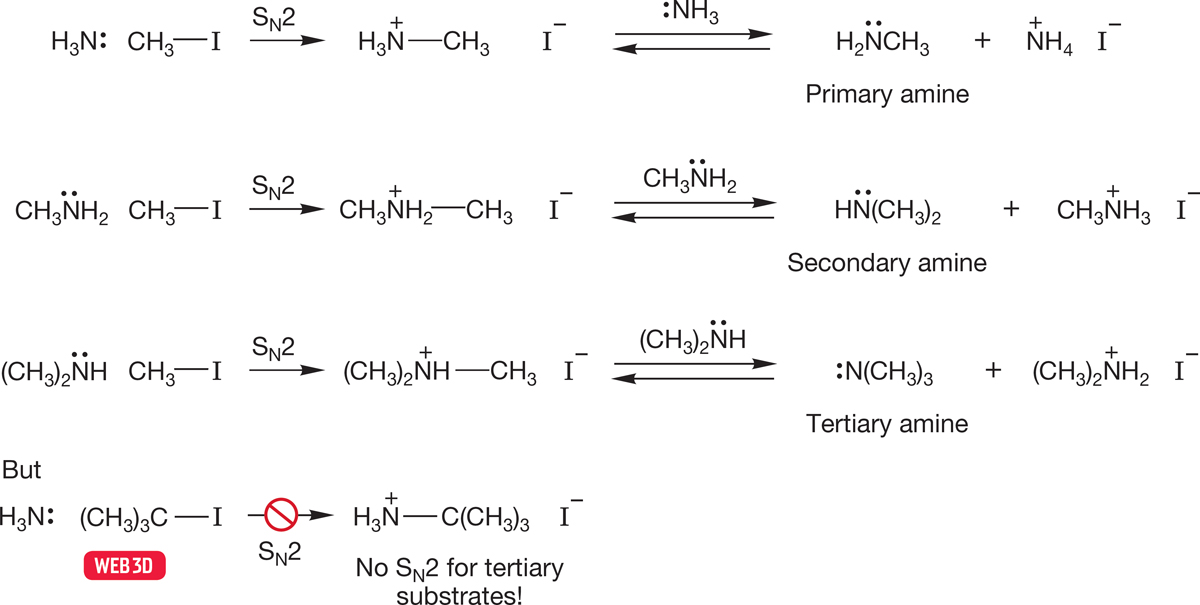
FIGURE 7.105 Alkylation reactions of amines through SN2 displacement followed by deprotonation.
Tertiary amines are also strong nucleophiles, and a final displacement reaction can occur to give a stable quaternary ammonium ion (Fig. 7.106). Thus, the alkylation process can be pushed to its logical conclusion in an effective synthesis of quaternary ammonium ions.
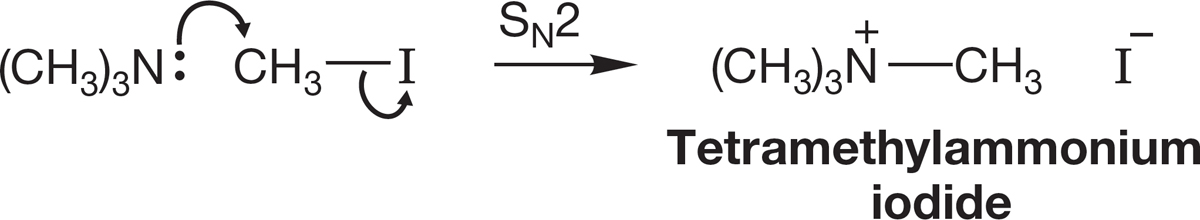
FIGURE 7.106 The formation of an ammonium ion through alkylation of a tertiary amine.
These alkylation reactions of amines are so efficient that it is difficult to stop at monoalkylation. However, Theodore Cohen (b. 1929) and his co-workers at the University of Pittsburgh worked out a method that uses ammonia in excess under pressure to generate primary amines efficiently. The vast excess of ammonia means that it is difficult for the initial alkylated product, methylamine, to compete with ammonia for a molecule of methyl iodide (Fig. 7.107).
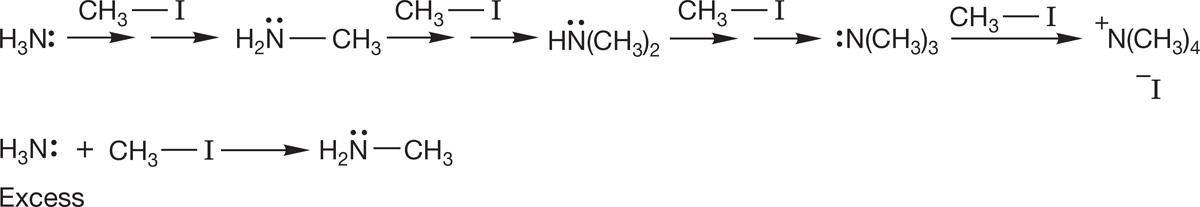
FIGURE 7.107 The synthesis of alkylamines through alkylation reactions (SN2 reactions).
PROBLEM 7.28 Predict the products for the following reactions:
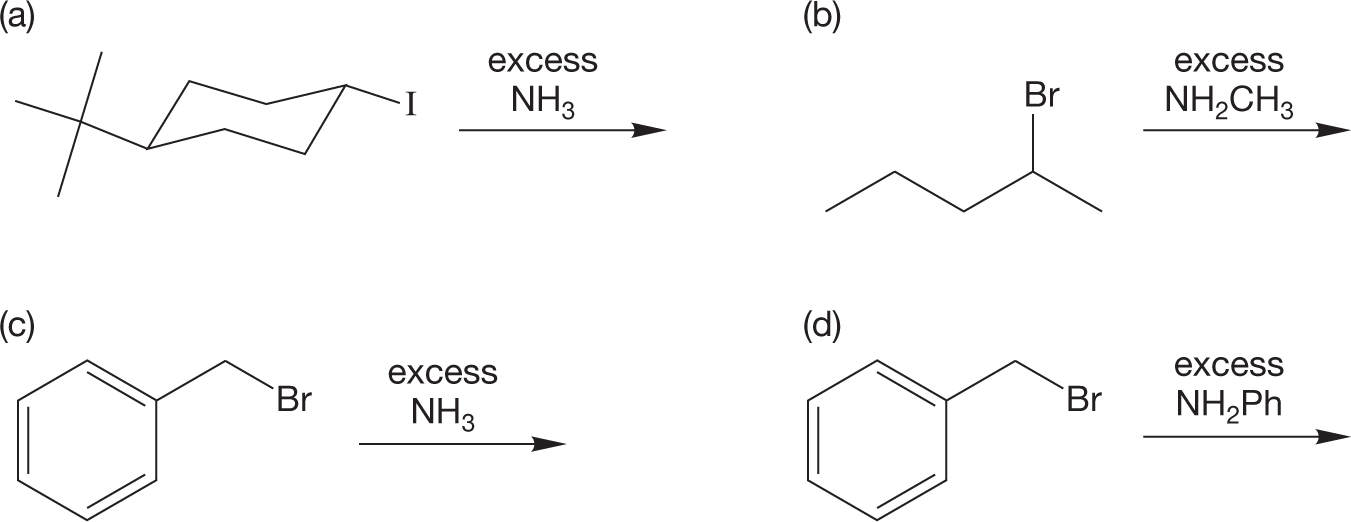
PROBLEM 7.29 Another nucleophilic synthesis of amines involves the reaction of amines with epoxides. This reaction is used to make epoxy glues. Write a mechanism for the reaction below.
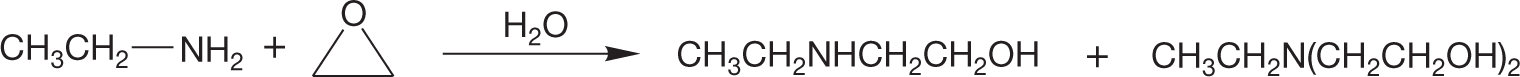
7.9d “Real-World” Difficulties In the real world, the challenges of synthesis are great—you not only need to find a reaction or, more likely, a sequence of reactions that will make the desired molecule, but also you must do so specifically and, in industry, economically. Specificity becomes increasingly important these days as we recognize how critical chirality is in biological action. Also, the more stereogenic centers (p. 156) a molecule has, the more difficult the synthesis. Imagine making a molecule with only a modest number of stereogenic atoms, say four. There are 16 possible isomers, and you almost certainly can use only one. Every reaction you use must lead to the correct stereochemistry and no others.
Although it is easy to look down your nose at questions of cost, you would be very wrong to do so. Chemists in industry must find sophisticated ways to make those stereochemically rich molecules without using superexpensive reagents that might be fine in very-small-scale work but utterly useless in work designed to produce a molecule to be used by millions of people. Doing clever syntheses with cheap reagents is much more difficult, both intellectually and practically, than making the molecule regardless of cost.
PROBLEM 7.30 Predict the product, if any, of the following reactions:
(a) HO− + CH3Br →
(b) HO− + CH3(CH2)3CH2Br →
(c) H2O + (CH3)3CBr →
(d) H2O + CH3(CH2)3CH2Br →
PROBLEM 7.31 Devise a synthesis of 5-bromo-2-pentyne. You may use any inorganic reagent, organic iodides containing up to four carbons, and the special reagents ethylene oxide (p. 237) and acetylene.
PROBLEM 7.32 Indicate which member of the following pairs of compounds will react faster in (1) the SN1 reaction and (2) the SN2 reaction. Explain your reasoning.
(a) 1-bromobutane and 2-bromobutane
(b) 1-chloropentane and cyclopentyl chloride
(c) 1-chloropropane and 1-iodopropane
(d) tert-butyl iodide and isopropyl iodide
(e) tert-butyl iodide and tert-butyl chloride
PROBLEM 7.33 Devise synthetic routes that will produce the following compounds as the major products from the indicated starting materials.
Figure 7.89 fails to show the potential complications we have talked about. There can be competition between the substitution reactions, SN1 and SN2, and the elimination reactions we will discuss in Chapter 8 are often a problem. Under some circumstances, the major products of the reactions summarized in Figure 7.89 will be alkenes. The identity of the leaving group, so conveniently avoided by using the symbol LG in the figure, makes a difference, and the structure of R, also deliberately vague in the figure, is vital. To design a successful synthesis, you have to think about all the components of the reaction.
PROBLEM SOLVING
Keeping track of synthetic methods can be difficult. You are right at the beginning of synthetic chemistry now, and here is a hint on how to keep track of it. Make a 4-inch × 6-inch file card for every structural type and then keep a short list of the available synthetic reactions on the card. To help in ordering things, and to keep as far as possible from the dreaded mindless memorization, you might make a mechanistic notation for each reaction as well. The reaction of acetylides with alkyl halides to make new acetylenes might get an “SN2 with primary substrates” beside it, for example. You can start your catalog with the reactions in Figure 7.89. Be sure to keep up to date; synthetic ability is the kind of thing that sneaks up on you, like whatever it was that worried Mr. Paige, as quoted at the beginning of this chapter. A sample file card for alkyl bromides is shown in Figure 7.108.
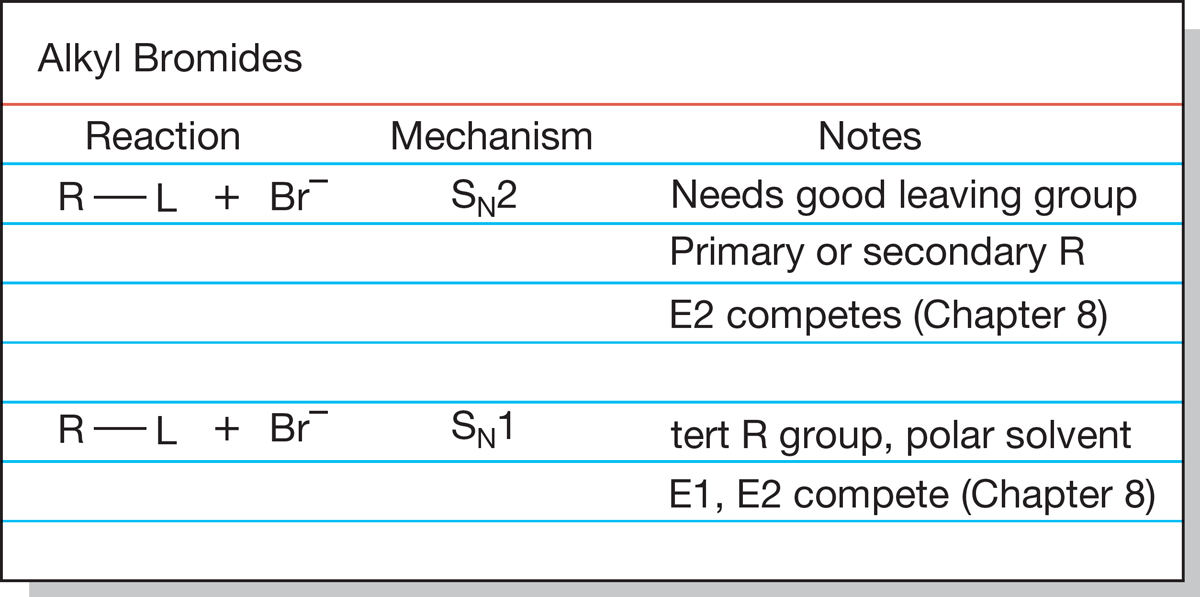
FIGURE 7.108 A sample file card for alkyl bromides.
PROBLEM 7.34 Use the following pKa data to decide whether sodium amide would be effective at producing vinyl anions  . pKa: ammonia, 38; vinyl hydrogen, ~46. Write the acid–base reaction for this process.
. pKa: ammonia, 38; vinyl hydrogen, ~46. Write the acid–base reaction for this process.