7.2 Review of Lewis Acids and Bases
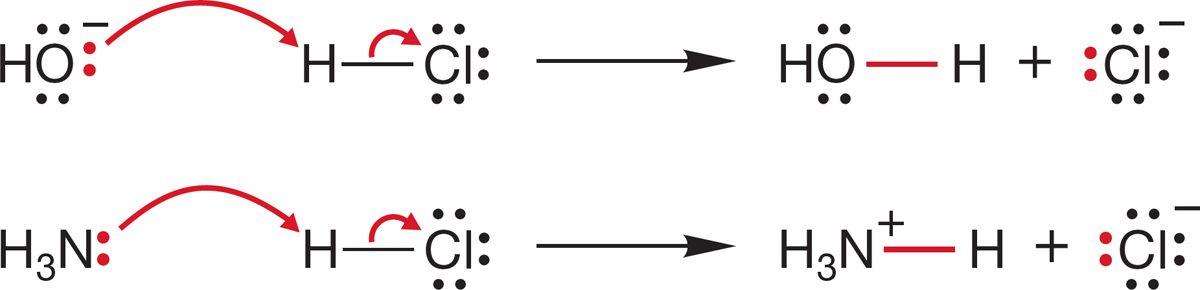
FIGURE 7.3 Two demonstrations of the application of the curved arrow formalism. We will see many other examples.
7.2a The Curved Arrow Formalism Recall that a most important bookkeeping technique, called the curved arrow formalism (p. 24), helps to sketch the broad outlines of what happens during a chemical reaction. However, it does not constitute a full reaction mechanism, which is a description of how the reaction occurs (p. 136). If a reaction mechanism is not an arrow formalism, what is it? A short answer would be a description, in terms of structure and energy, of the intermediates and transition states separating starting material and product. Implied in this description is a picture of how the participants in the reaction must approach each other. The arrow formalism is a powerful device of enormous utility in helping to keep track of bond makings and breakings. In this formalism, chemical bonds are shown as being formed from pairs of electrons flowing from a donor toward an acceptor, often, but not always, displacing other electron pairs. Figure 7.3 shows two examples. In the first, hydroxide ion, acting as a Brønsted base, displaces the pair of electrons in the hydrogen–chlorine bond. The reaction of ammonia (:NH3) with hydrogen chloride is another example in which the electrons on neutral nitrogen are shown displacing the electrons in the hydrogen–chlorine bond.
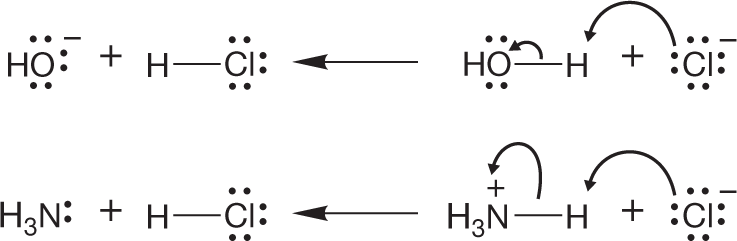
WORKED PROBLEM 7.1 Draw arrow formalisms for the reversals of the two reactions in Figure 7.3.
ANSWER This problem may seem to be routine, but it really is not. The key point here is to begin to see all reactions as proceeding in two directions. Nature doesn’t worry about “forward” or “backward.” Thermodynamics dictates the direction in which a reaction will run.
7.2b Lewis Acids and Bases In Sections 1.8 and 2.15, we began a discussion of acids and bases that will last throughout this book. Then we spent some time in Chapter 3 reviewing Brønsted acids and bases in the context of the addition reactions and extended the subject of acidity and basicity to Lewis acids and Lewis bases. These concepts will hold together much of the material in the remaining chapters.
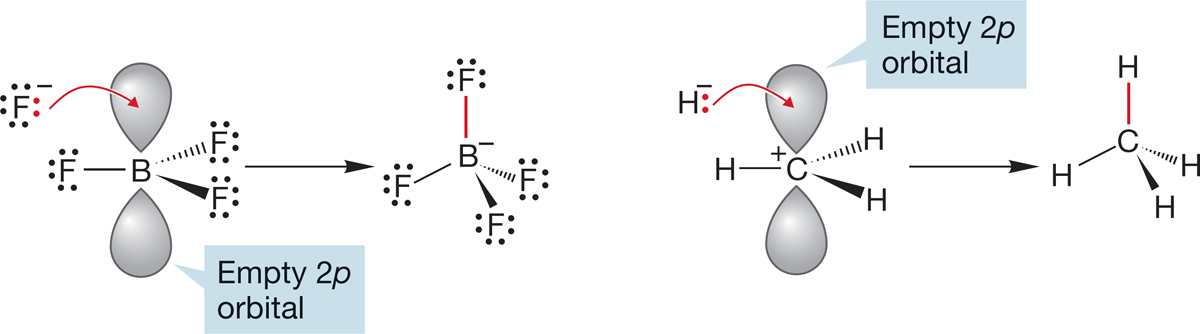
FIGURE 7.4 The fluoride and hydride ions are Lewis bases reacting with the Lewis acids BF3 and +CH3.
Consider two conceptually related processes: the reaction of boron trifluoride with fluoride ion, F−, and the reaction of the methyl cation with hydride, H:− (Fig. 7.4).
In these two reactions, no proton is being donated or accepted, so by a strict definition of Brønsted acids and bases we are not dealing with acid–base chemistry. As we already know from Chapter 3, however, a more general definition of acids and bases does deal with this situation. A little review may be helpful: A Lewis base is defined as any species with a reactive pair of electrons (an electron donor). This definition may seem very general, and indeed it is. It gathers under the “base” category all manner of odd compounds that don’t really look like bases. The key word that confers all this generality is reactive. Reactive under what circumstances? Almost everything is reactive under some set of conditions. And that’s true; compounds that are extraordinarily weak Brønsted bases can still behave as Lewis bases.
If the definition of a Lewis base seems general and perhaps vague, the definition of a Lewis acid is even worse. A Lewis acid is anything that will react with a Lewis base (thus, an electron acceptor). Therein lies the power of these definitions. They allow us to generalize—to see as similar reactions those that on the surface appear very different.
To describe Lewis acid–Lewis base reactions in orbital terms, we need only point out that a stabilizing interaction of orbitals involves the overlap of a filled orbital (Lewis base) with an empty orbital (Lewis acid) (Fig. 7.5).
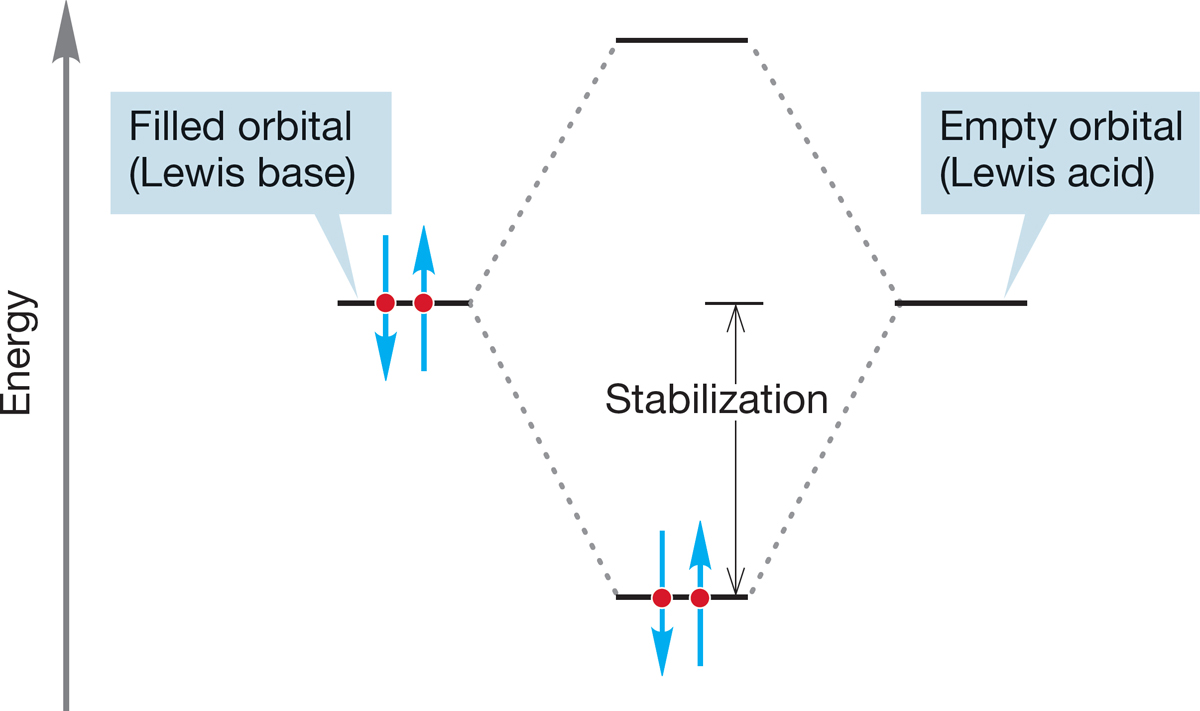
FIGURE 7.5 The interaction of a filled orbital with an empty orbital is stabilizing.
In the examples in Figure 7.4, the Lewis bases are the fluoride and hydride ions. In fluoride, the electrons occupy a 2p orbital, and in hydride they occupy a 1s orbital. The Lewis acids are boron trifluoride and the methyl cation, each of which bears an empty 2p orbital. A schematic picture of these interactions is shown in Figure 7.6.
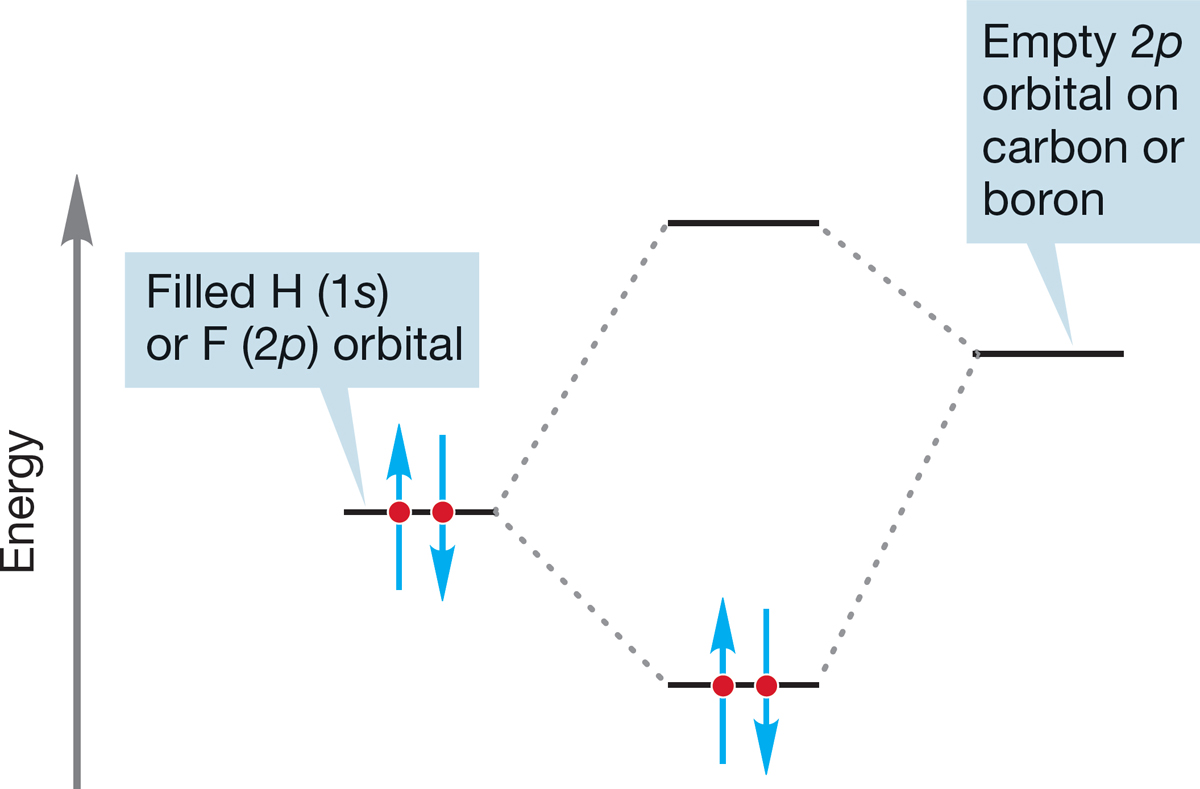
FIGURE 7.6 A schematic interaction diagram for the reactions of Figure 7.4.
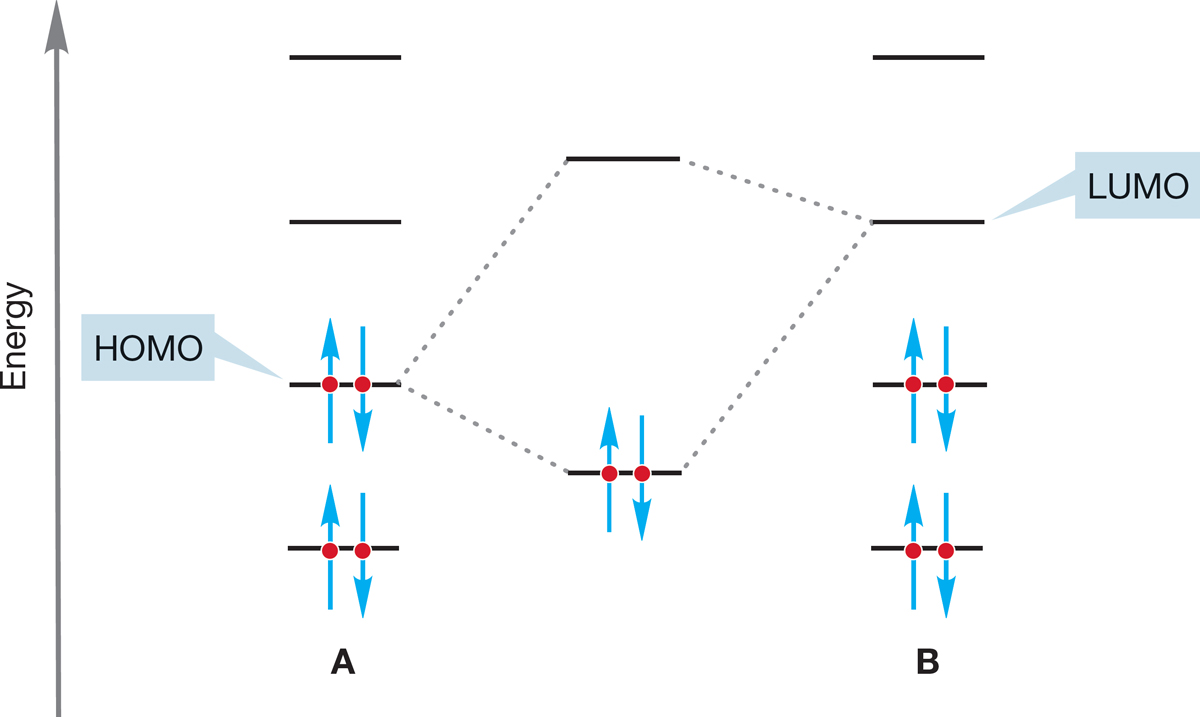
FIGURE 7.7 In two typical molecules, the strongest stabilizing interaction is likely to be between the HOMO of one molecule (A) and the LUMO of the other molecule (B) because the strongest interactions are between the orbitals closest in energy.
7.2c HOMO–LUMO Interactions It is possible to elaborate a bit on the generalization that “Lewis acids (electrophiles) react with Lewis bases (nucleophiles)” by realizing that the strongest interactions between orbitals are between the orbitals closest in energy. For stabilizing filled–empty overlaps, this will almost always mean that we must look at the interaction of the highest occupied molecular orbital (HOMO) of one molecule (A), which is the nucleophile, with the lowest unoccupied molecular orbital (LUMO) of the other (B), which is the electrophile (Fig. 7.7).
In the reaction of BF3 with fluoride, it is the filled fluorine 2p orbital that is the HOMO and the empty 2p orbital on boron that is the LUMO (Fig. 7.8). This nucleophile plus electrophile reaction forms a new B―F bond to produce BF4−. All Lewis acid–Lewis base reactions can be described in similar terms.
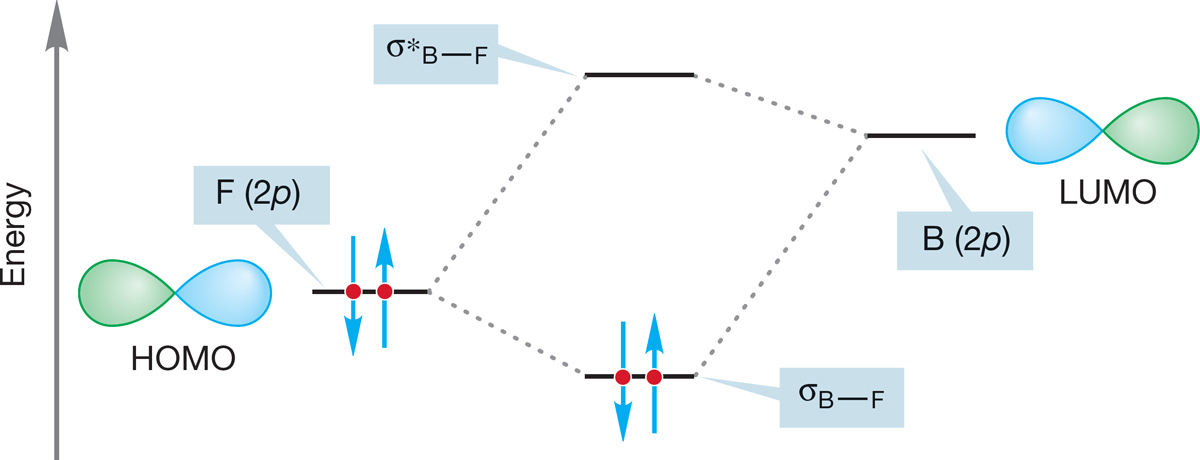
FIGURE 7.8 The interaction of the LUMO for BF3 (an empty 2p orbital on boron) and the HOMO of F – (a filled 2p orbital on fluorine) produces two new molecular orbitals: an occupied bonding σ orbital and an unoccupied antibonding σ* orbital.
PROBLEM 7.2 Identify the HOMO and LUMO in the reaction of the methyl cation with hydride in Figure 7.4.
WORKED PROBLEM 7.3 Point out the HOMO and LUMO in the following reactions:
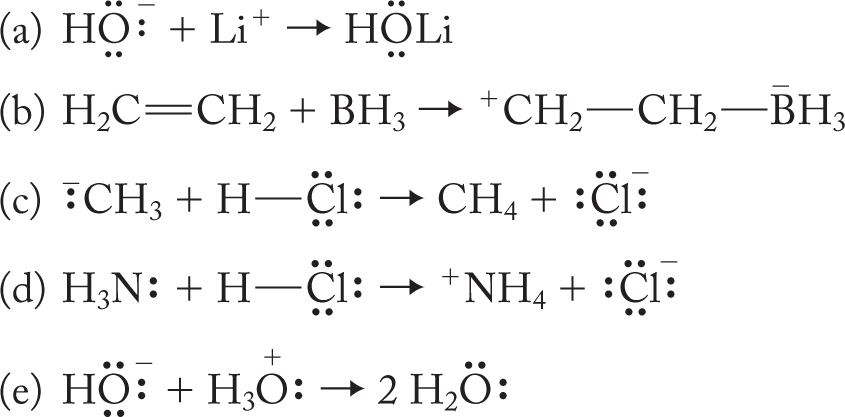
ANSWER
(a) HOMO: Filled nonbonding orbital on hydroxide
LUMO: Empty 2s orbital on Li+
(b) HOMO: Filled π orbital of ethylene
LUMO: Empty 2p orbital on BH3
(c) HOMO: Filled sp3 nonbonding orbital of methyl anion
LUMO: Empty σ* orbital of H―Cl
(d) HOMO: Filled sp3 nonbonding orbital of ammonia, :NH3
LUMO: Empty σ* orbital of H―Cl
(e) HOMO: Filled nonbonding orbital of hydroxide
LUMO: Empty σ* orbital of an O―H bond of H3O+