2.15 Acids and Bases Revisited: More Chemical Reactions

FIGURE 2.58 The two Brønsted bases, HO− and Cl−, compete for the proton, H+.
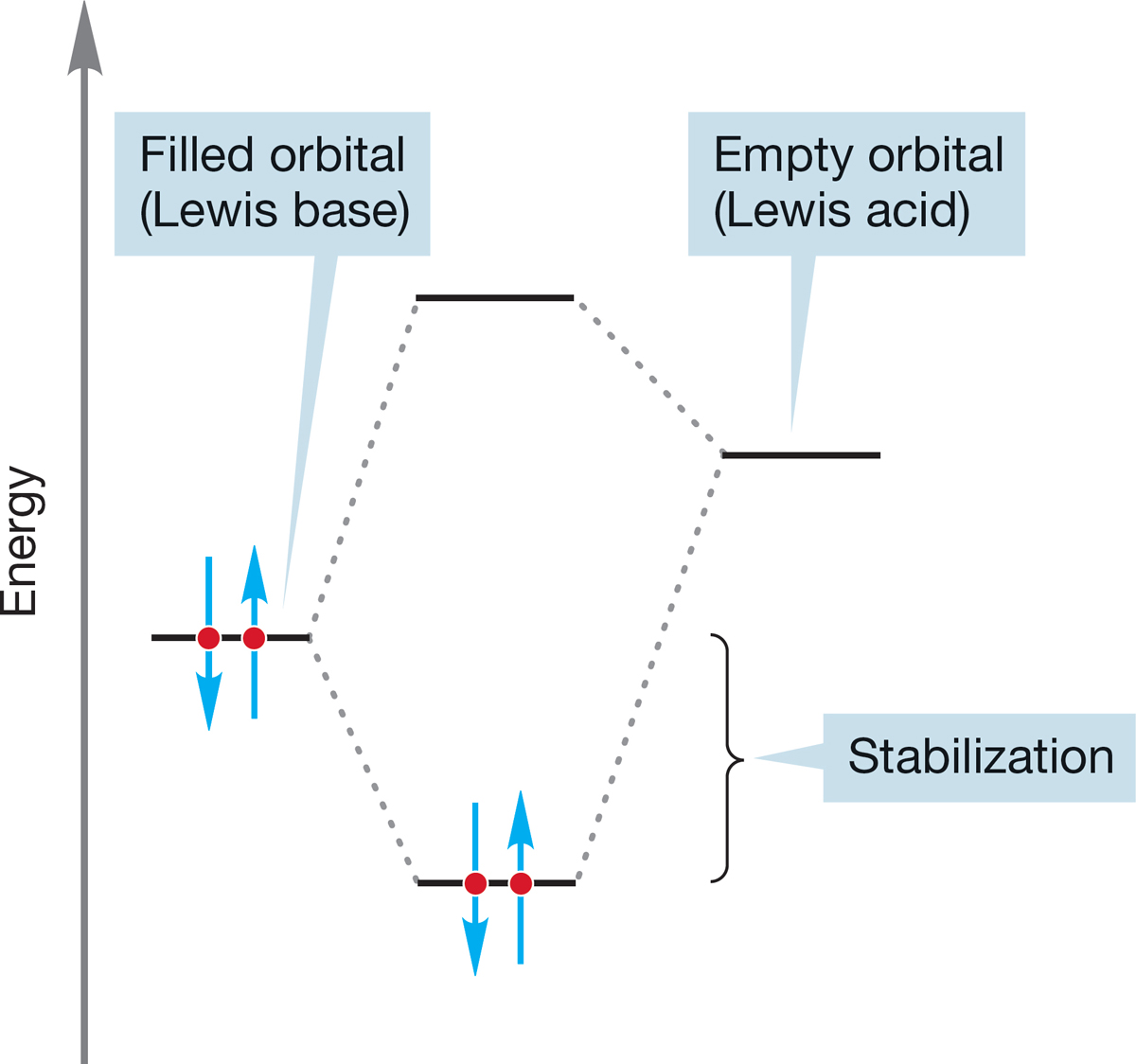
FIGURE 2.59 The interaction of a filled orbital (Lewis base) with an empty orbital (Lewis acid) is stabilizing.
In Section 1.7 (p. 37), we introduced acids and bases. Now we know quite a bit more about structure and can return to the important subject of acids and bases in greater depth. In particular, we know about carbocations and carbanions, which play an important role in acid–base chemistry in organic chemistry. The Lewis definition of acids and bases is far more inclusive than the Brønsted–Lowry6 definition, which focuses solely on proton donation (Brønsted–Lowry acid) and acceptance (Brønsted–Lowry base). The archetypal Brønsted acid–base reaction is the reaction between KOH and HCl to transfer a proton from HCl to HO−. This reaction is a competition between the hydroxide and the chloride for a proton. In this case, the stronger base hydroxide wins easily (Fig. 2.58).
In the Lewis description of acids and bases, any reaction between an empty orbital and a reactive pair of electrons in a filled orbital is an acid–base reaction. Another way of putting this is to point out that the interaction between an empty orbital (Lewis acid) and a filled orbital (Lewis base) is stabilizing (Fig. 2.59).
Consider the methyl cation (Fig. 2.60). That empty 2p orbital makes +CH3 a powerful Lewis acid, very reactive toward all manner of Lewis bases with their electron pairs ready to react. Figure 2.60 gives three examples of such Lewis acid–Lewis base reactions, each of which fits the orbital interaction diagram of Figure 2.59.
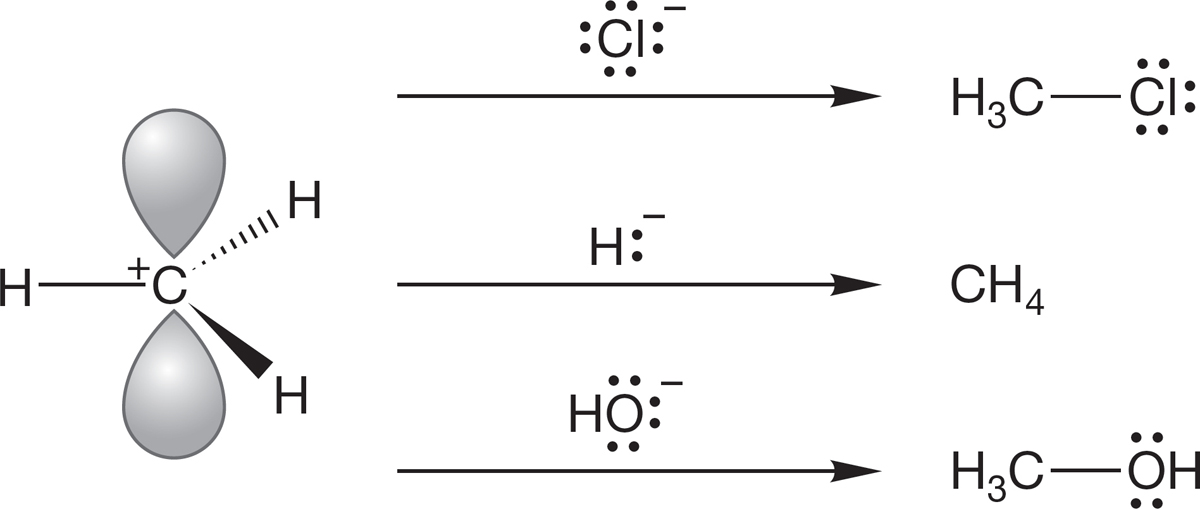
FIGURE 2.60 Three Lewis bases reacting with the methyl cation, a Lewis acid.
In Figure 2.61, the curved arrow formalism (p. 24) shows the electron flow as the new bond is formed in this illustrated nucleophile–electrophile (Lewis base–Lewis acid) reaction.
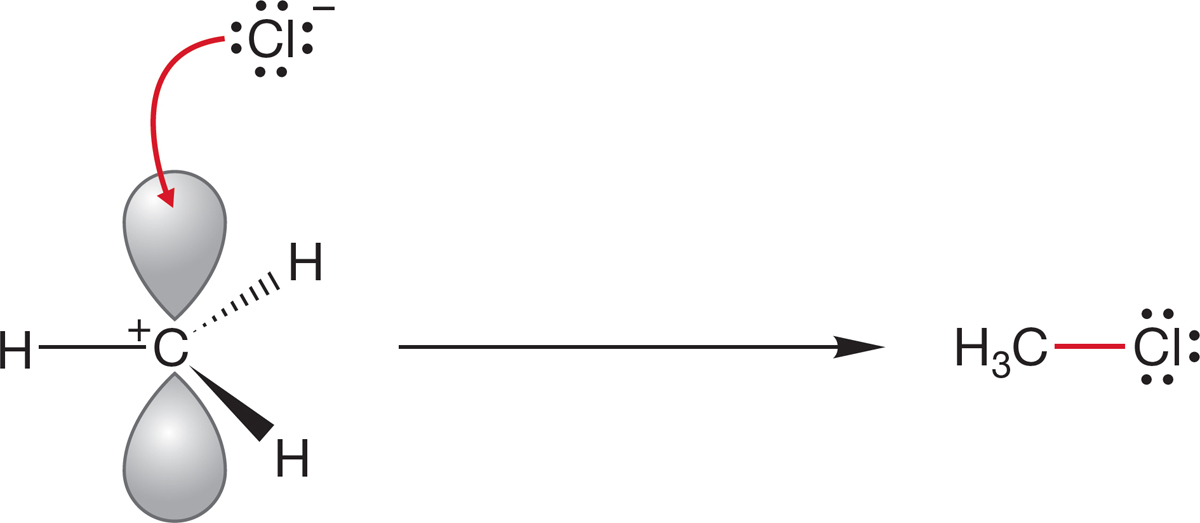
FIGURE 2.61 The curved arrow formalism for the formation of methyl chloride from the methyl cation (electrophile) and a chloride ion (nucleophile).
WORKED PROBLEM 2.40 Write curved arrow formalisms and products for the following reactions. Identify the participants as acids and bases. Sketch in the empty orbital for each Lewis acid, and fill in the complete Lewis dot structures for the Lewis bases. Remember that charge must be conserved in each of these reactions.
(a) (CH3)3C+ + Br−➔
(b) (CH3)3C+ + NH3➔
(c) H+ + −NH2➔
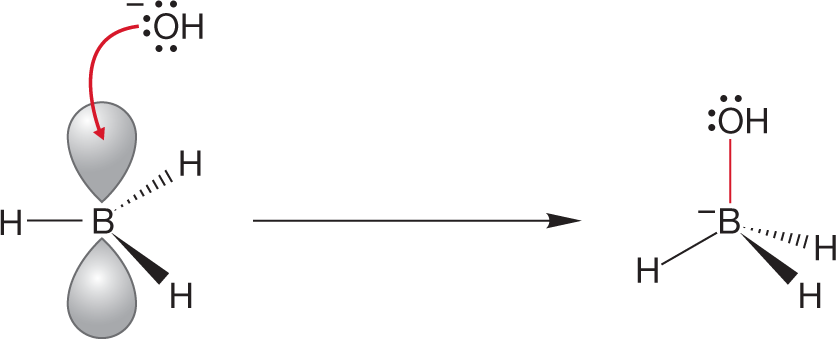
*(d) H3B + HO−➔
ANSWER (d) Hydroxide must be the Lewis base, but where is the empty orbital? Where is the Lewis acid? Recall the structure of the sp2-hybridized borane (p. 59). Boron is neutral, but there is an empty 2p orbital on boron, and borane is very definitely a Lewis acid. Reaction with the Lewis base hydroxide looks just like the reaction of the methyl cation with hydroxide (Fig. 2.60) except that the product is negatively charged.
Reactions between electrophiles and nucleophiles, even the very simple ones, form the basis for understanding much of organic chemistry. Essentially all polar reactions can be looked at in exactly these terms—the ultimately stabilizing reaction of a filled orbital with an empty orbital. Figure 2.59 really does describe much of the next 1000 pages or so! But, of course, the details will change, and there are surely devils in those details. Those all-too-prolific details will be easier to manage if you keep Figure 2.59 in mind at all times—search for the Lewis base and the Lewis acid in every reaction, and you will be able to generalize.
6These acids and bases were named after Johannes Nicolaus Brønsted (1879–1947) and Thomas Martin Lowry (1874–1936).