7.11 Additional Problems
This chapter expands the topic of organic synthesis. We still have very few reactions for making molecules at our disposal—basically just HX addition to alkenes, acid-catalyzed H2O addition to alkenes, substitution of an R―LG using the SN1 and SN2 substitutions of R―LG. Nonetheless, it is possible to devise simple routes to target molecules and even to craft a few multistep sequences. Organic synthesis is one of the most creative (and fun) parts of this science, and so it is important to start early. Here, we group several synthesis-related problems before going to more mechanistic material. In just a few chapters, we will have many more reactions under control and be able to do much fancier work. Stay tuned.
PROBLEM 7.35 The SN2 reaction that occurs between an alkyl halide and a nucleophile is a very important process in organic chemistry. For this reason, it is necessary to recognize the nucleophilicity of various reagents. In the following pairs of compounds, indicate the more nucleophilic reagent. Explain your reasoning.
(a) (CH3CH2)3N (CH3CH2)3P
(b) (CH3CH2)3N (CH3CH2)2N−
(c) CH3CH2SNa CH3CH2OK
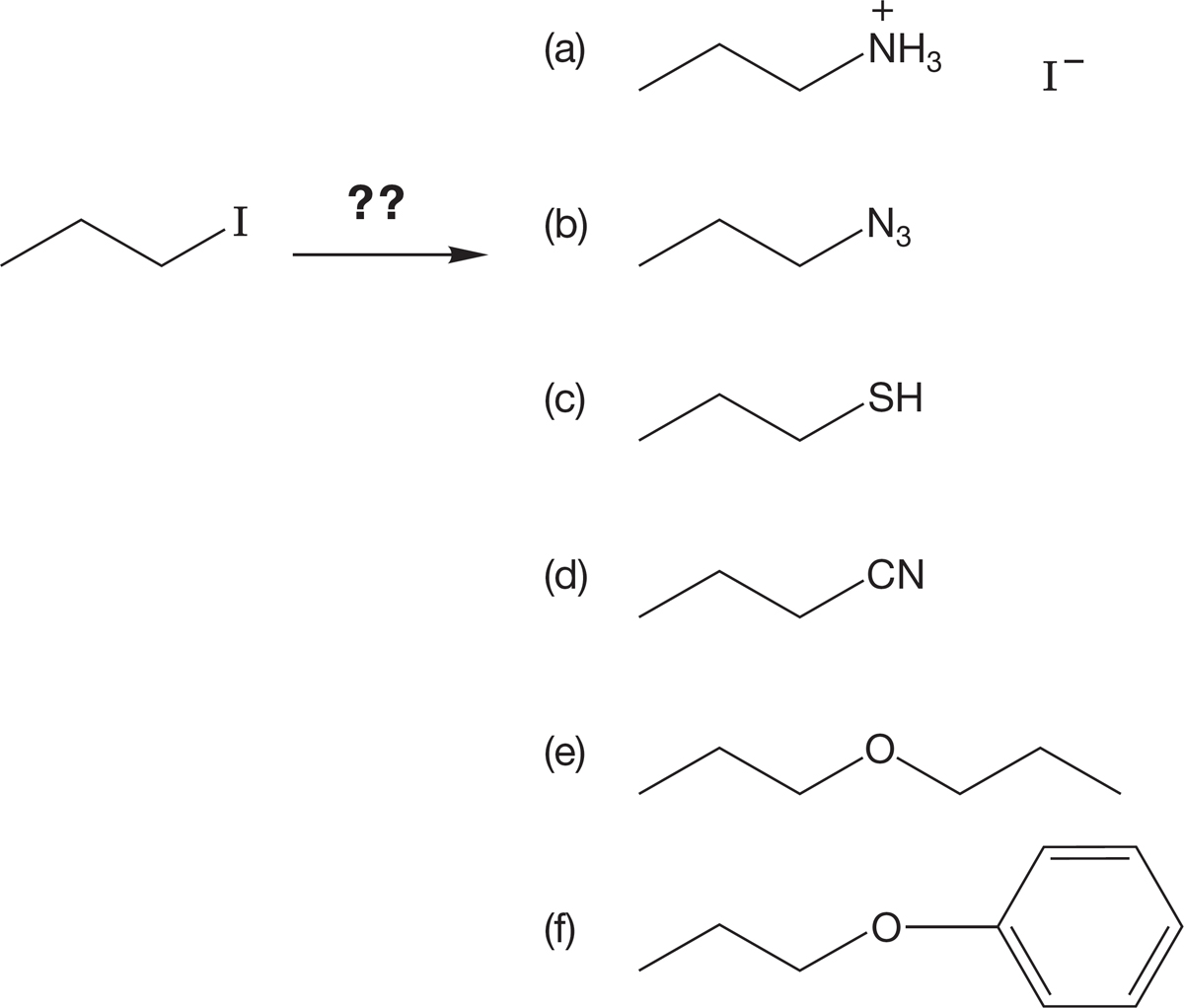
PROBLEM 7.36 Which nucleophiles would serve to effect the following conversions of 1-iodopropane?
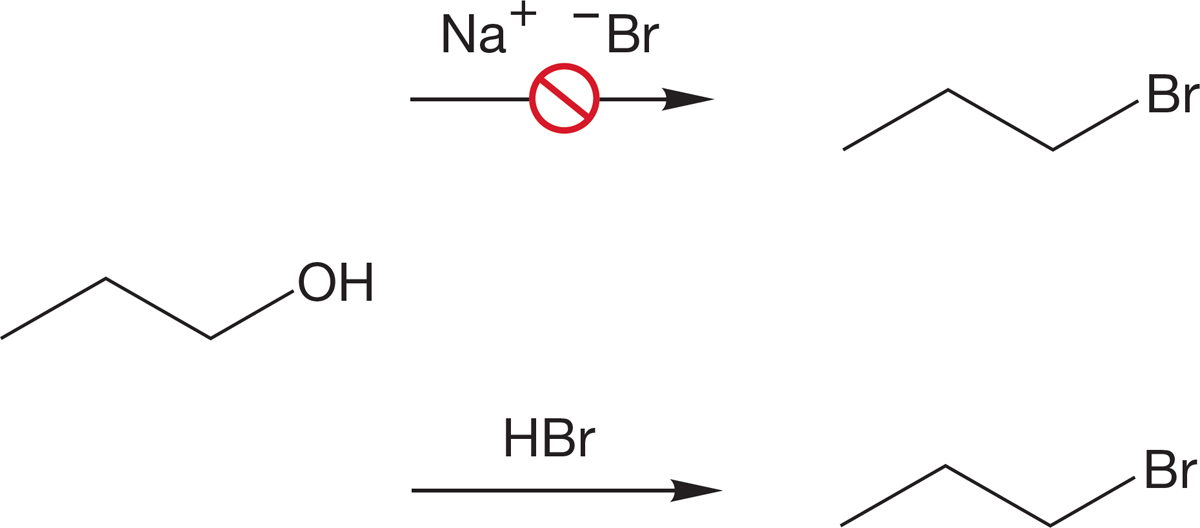
PROBLEM 7.37 Explain with crystal-clear clarity why treatment of 1-propanol with NaBr fails to give 1-bromopropane, but treatment of 1-propanol with HBr does give 1-bromopropane.
PROBLEM 7.38 Propose a synthesis of each of the following compounds starting with 1-butene and any other reagents:
(a) 2-chlorobutane
(b) 2-methoxybutane
(c) 2-butanamine
(d) butane
PROBLEM 7.39 Propose a synthesis of each of the following compounds starting with 2-butanol and any other reagents:
(a) 2-chlorobutane
(b) 2-methoxybutane
(c) 2-butanamine
(d) 2-butanethiol
PROBLEM 7.40 Why are sulfonates (p. 299) such good participants in the SN1 and SN2 reactions?
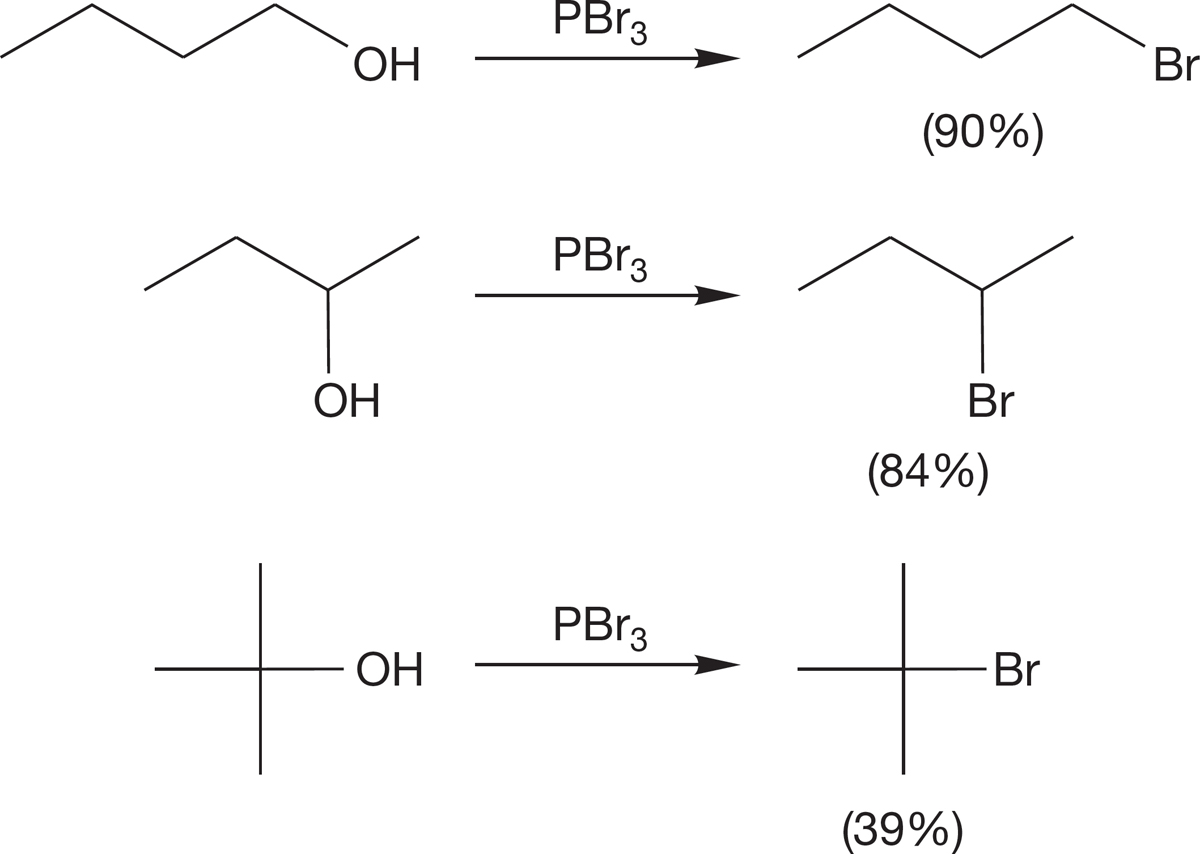
PROBLEM 7.41 Write a mechanism for the formation of 1-bromopropane from the reaction of 1-propanol and PBr3.
PROBLEM 7.42 You may be familiar with the compound tert-butyl methyl ether. Its common acronym is MTBE. It has been an additive for gasoline since 1979, although its use has declined in the United States since 2003. How would you make MTBE if you have bottles of tert-butyl alcohol and methyl alcohol? You may use any other inorganic reagents needed. Your proposed route should avoid mixtures containing undesired products.
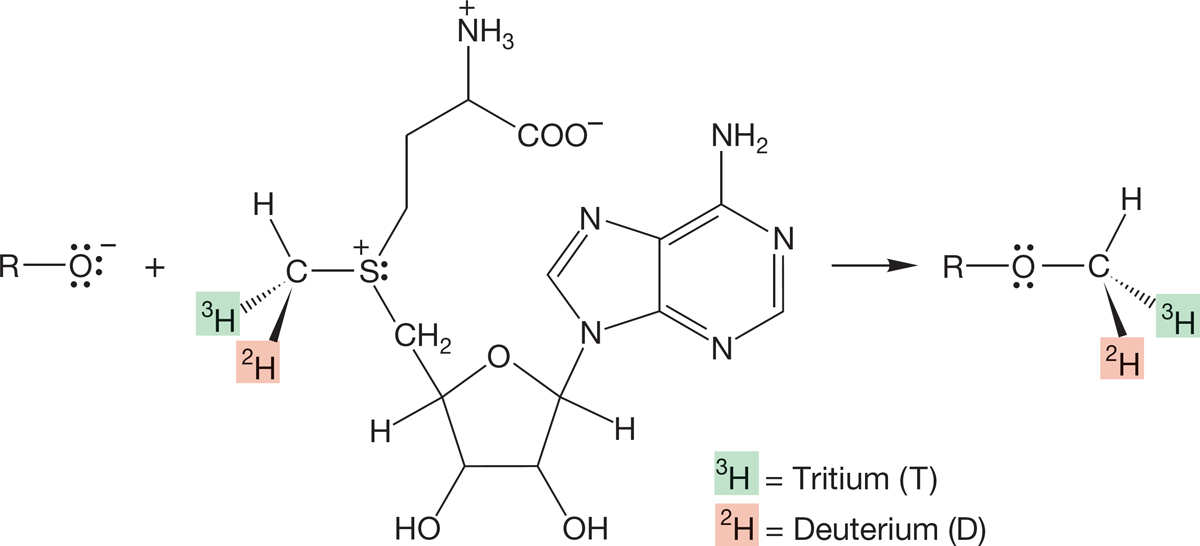
PROBLEM 7.43 The reaction shown in the following figure was used to help clarify the SN2 nature of the alkylation reactions of S-adenosylmethionine. Explain what the results tell us about the mechanism.
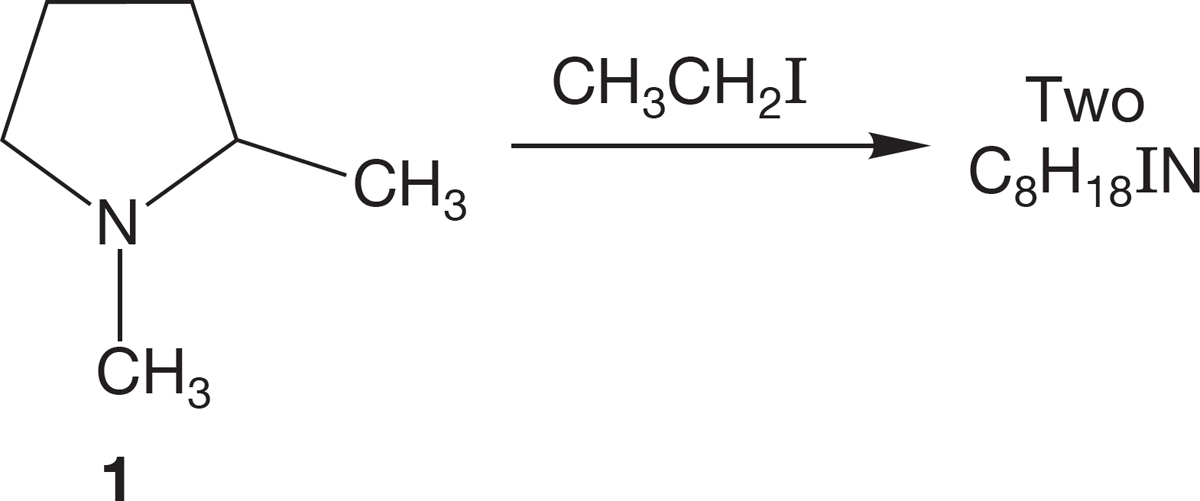
PROBLEM 7.44 (a) Reaction of 1,2-dimethylpyrrolidine (1) with ethyl iodide leads to two isomeric ammonium ions, C8H18IN. Explain.
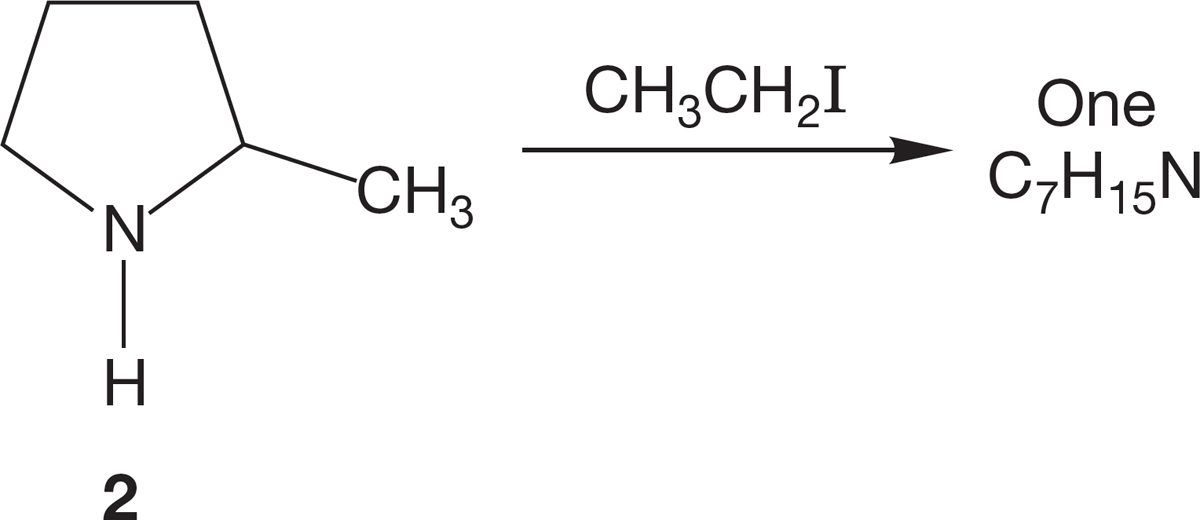
(b) However, when 2-methylpyrrolidine (2) undergoes a similar reaction with ethyl iodide, followed by treatment with weak base, analysis of the product by 1H NMR spectroscopy reveals only one set of signals for C7H15N. Explain.
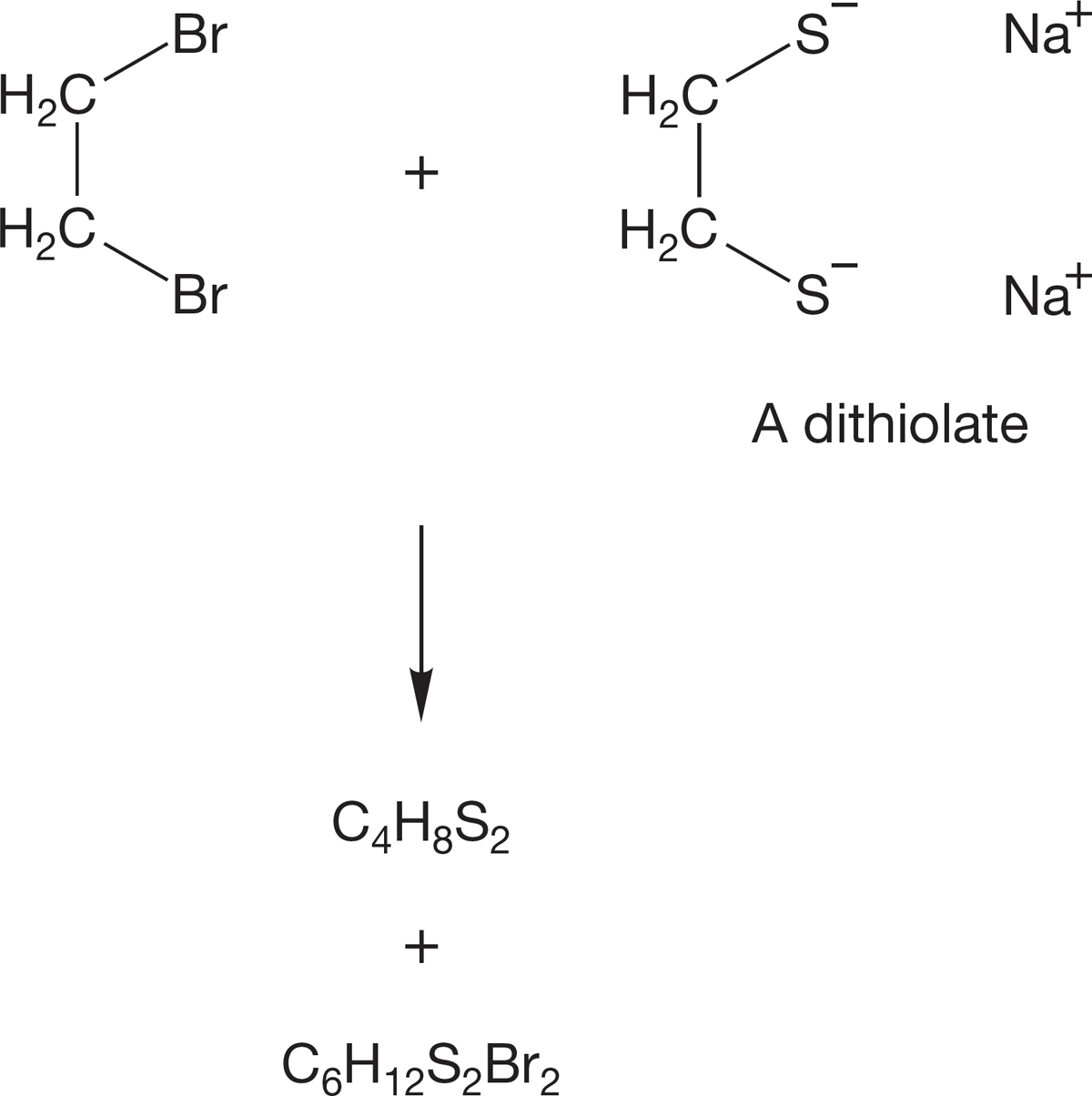
PROBLEM 7.45 Treatment of 1,2-dibromoethane with the dithiolate shown in the following figure leads to two products, C4H8S2 and C6H12S2Br2. Write structures for these products and explain how they are formed.
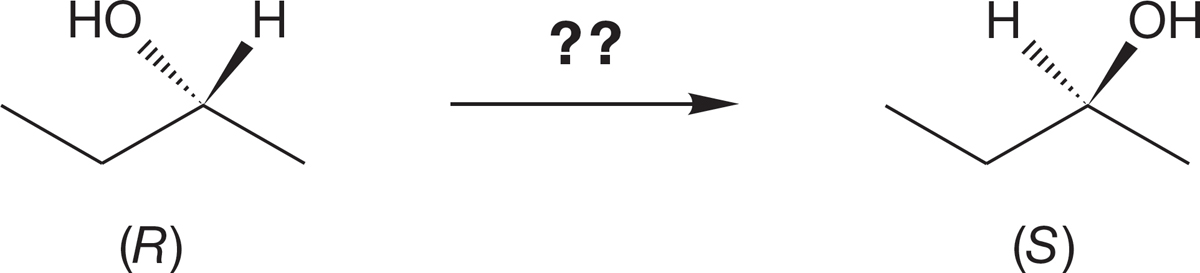
PROBLEM 7.46 Devise a synthetic route that will convert (R)-sec-butyl alcohol into (S)-sec-butyl alcohol.
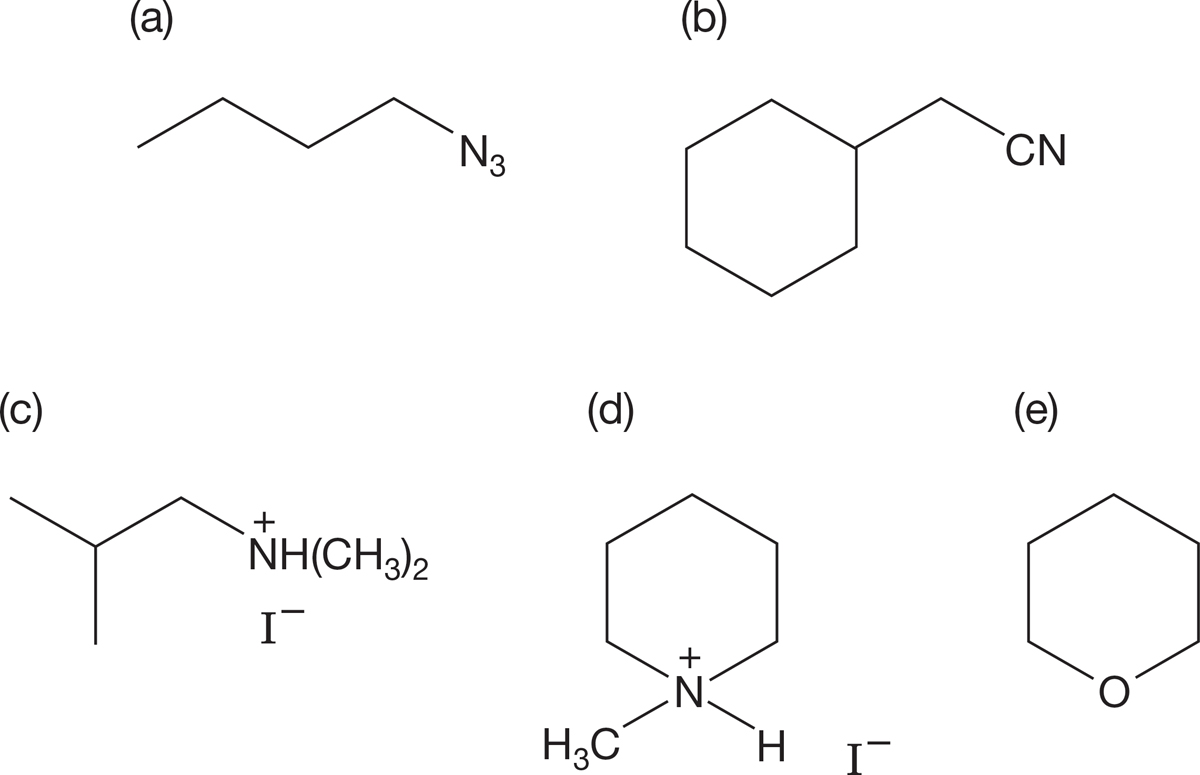
PROBLEM 7.47 Devise SN2 reactions that would lead to the following products:
PROBLEM 7.48 Predict the products of the following SN2 reactions:
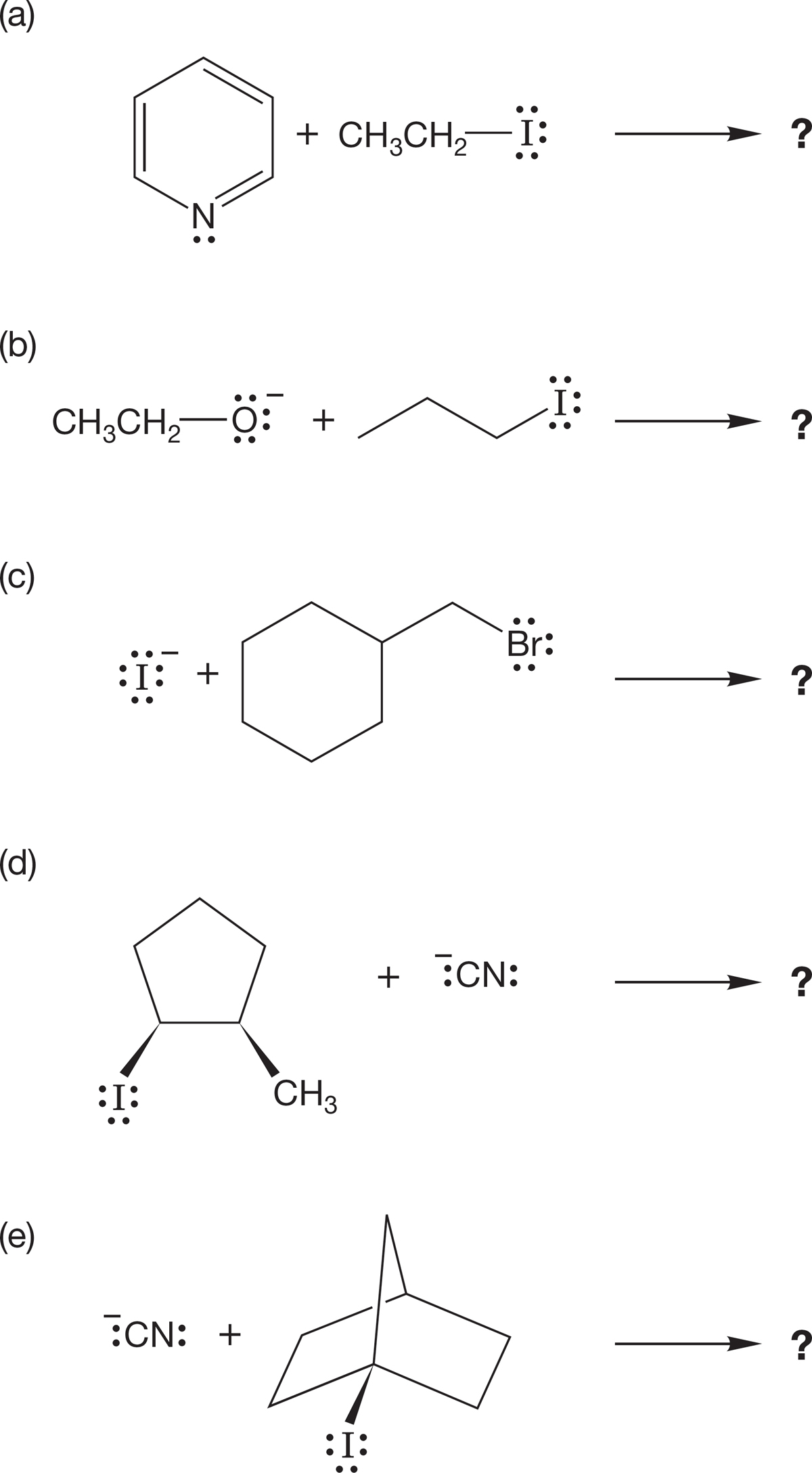
PROBLEM 7.49 Treatment of 1-ethoxybutane with HI leads to both butyl iodide and ethyl iodide, along with the related alcohols. By contrast, when tert-butyl ethyl ether is treated the same way, only ethyl iodide and tert-butyl alcohol are formed. tert-Butyl iodide and ethyl alcohol are not produced. Explain.
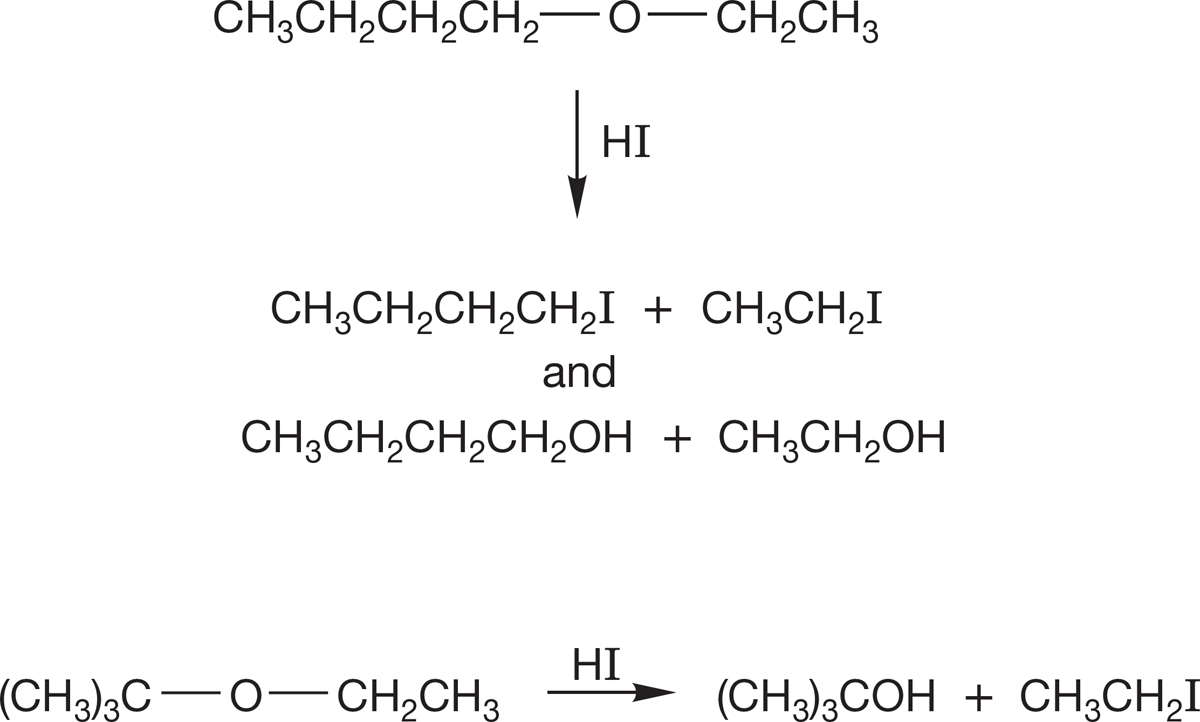
PROBLEM 7.50 Predict the products of the following SN1 reactions:
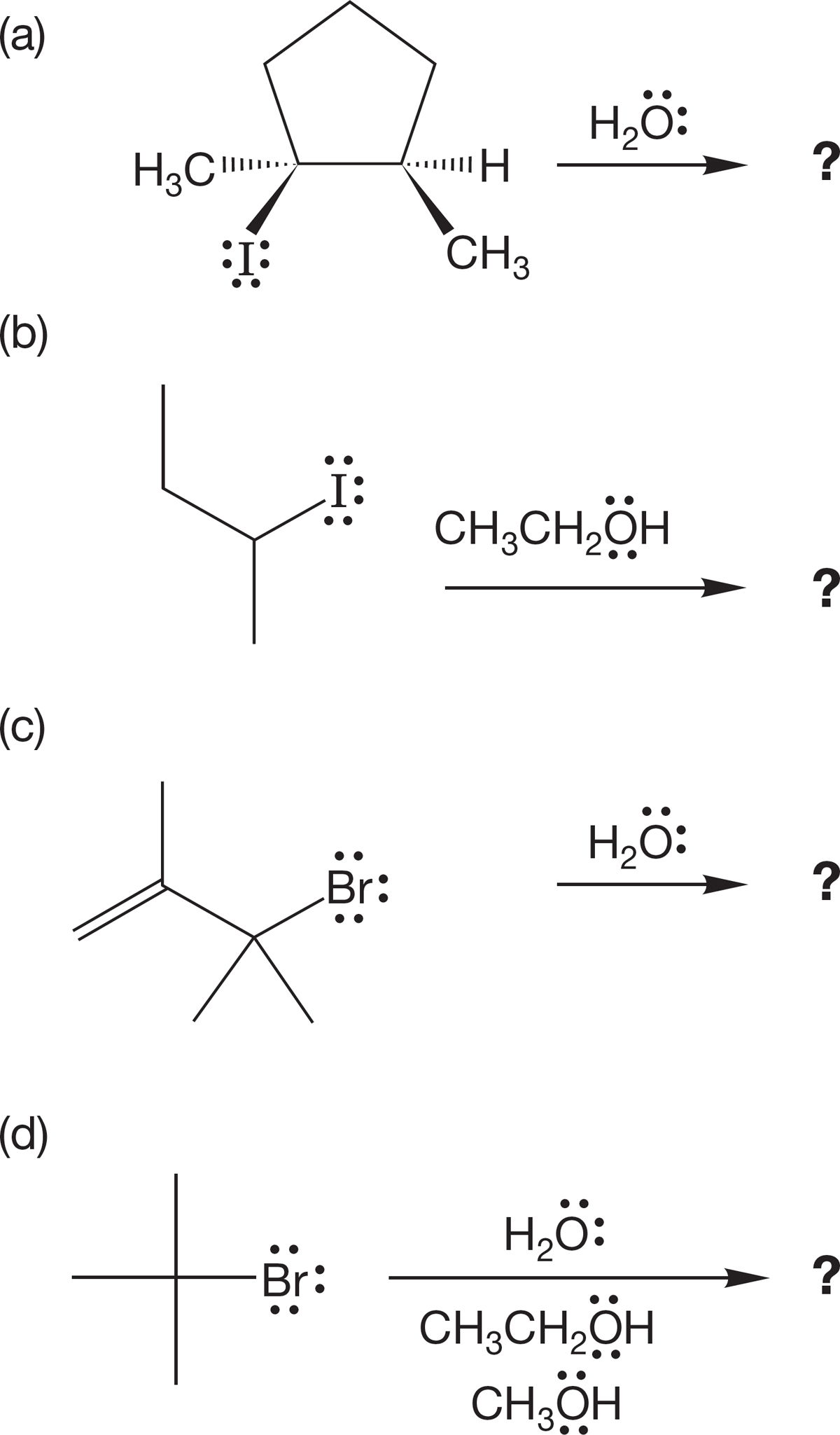
PROBLEM 7.51 You are given a supply of ethyl iodide, tert-butyl iodide, sodium ethoxide, and sodium tert-butoxide. Your task is to use the SN2 reaction to make as many different ethers as you can. In principle, how many are possible? In practice, how many can you make?
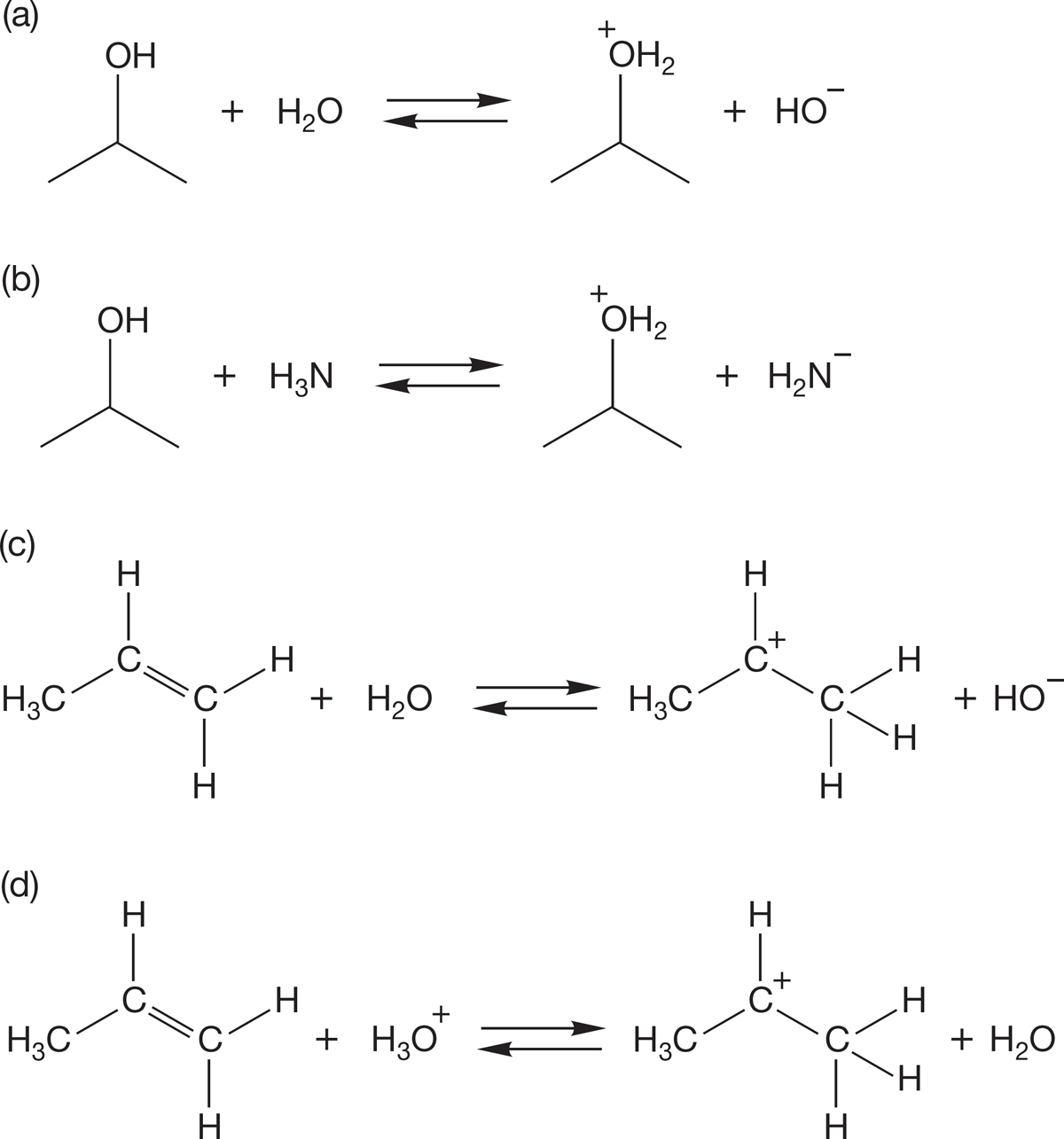
PROBLEM 7.52 Indicate which direction the equilibrium reactions favor for each example shown. Use the pKa values on the inside back cover of the book to confirm your answers. Is it practical to protonate an alcohol with water? Is it practical to protonate an alkene with water? A carbocation has a pKa ~ −11.
PROBLEM 7.53 Explain why a sulfonate (p. 299) is a better leaving group than an acetate (OCOR).
PROBLEM 7.54 Write arrow formalisms for the following reactions:
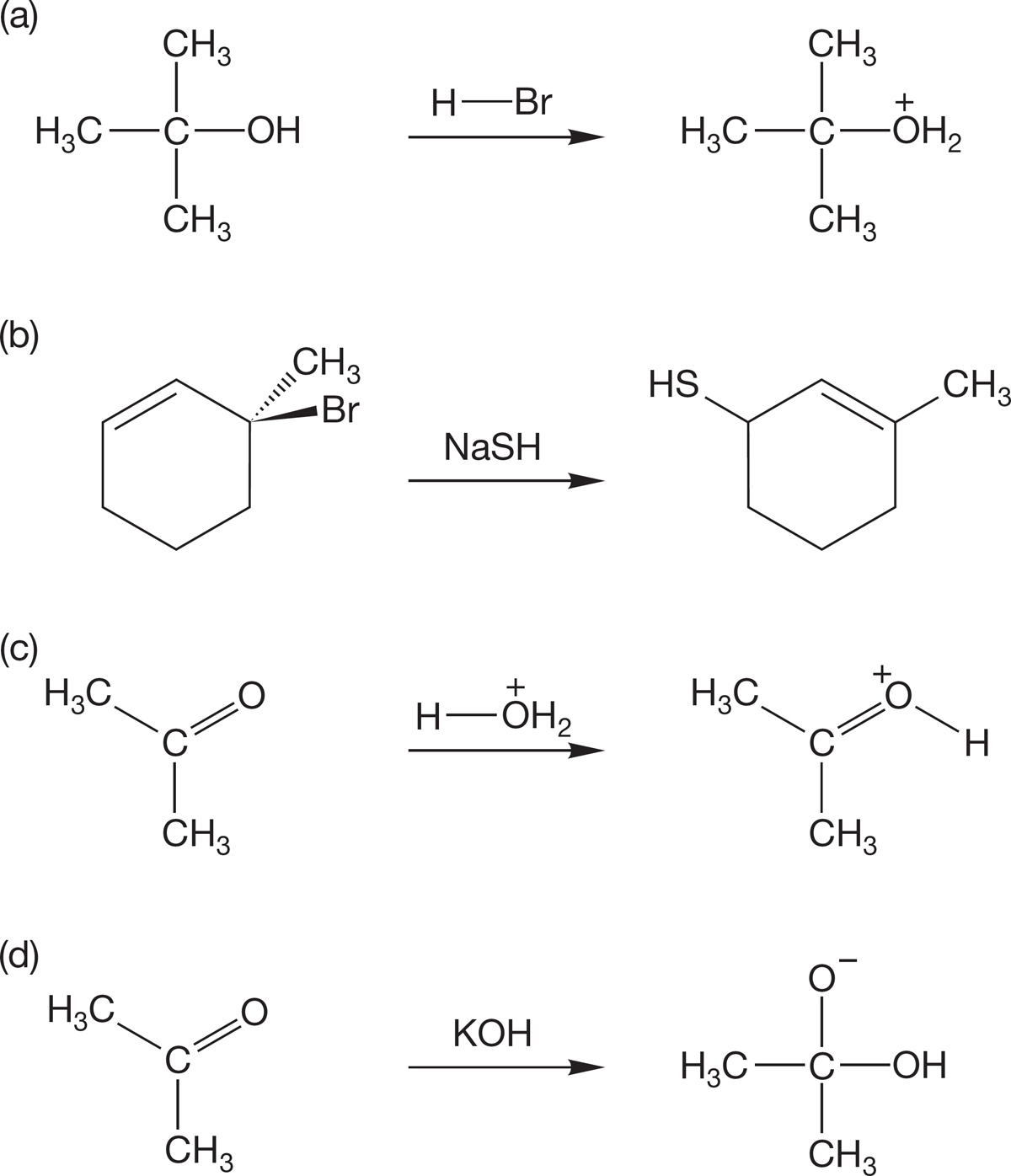
PROBLEM 7.55 SN2 reactions at the allyl position are especially facile. For example, allyl compounds are even more reactive than methyl compounds (Table 7.3, p. 291). Explain this rate difference. Why are allyl compounds especially fast? Hint: This, like all “rate” questions, can be answered by examining the structures of the transition states involved.
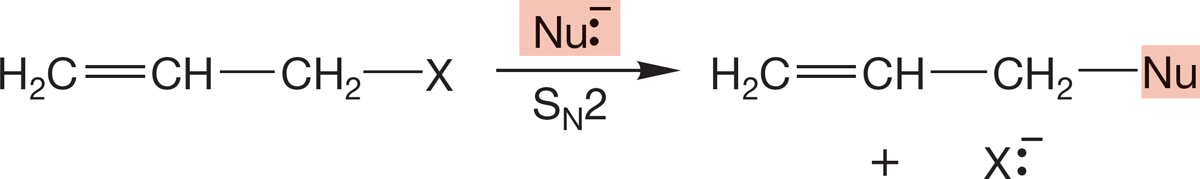
PROBLEM 7.56 Circle the molecule in each pair that will react more rapidly in an SN2 reaction. Explain your reasoning.
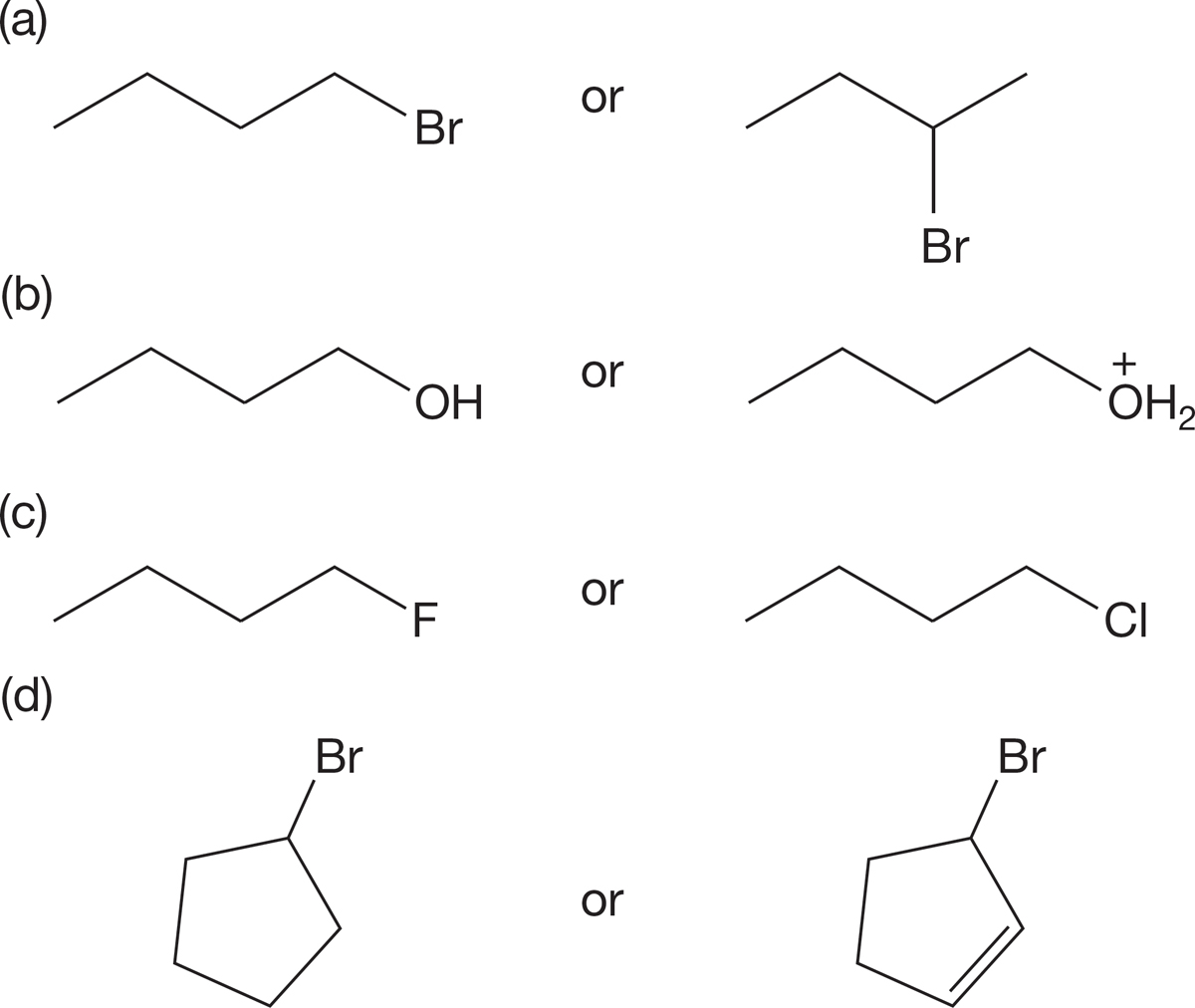
PROBLEM 7.57 Circle the molecule in each pair that will react more rapidly in an SN1 reaction. Explain your reasoning.
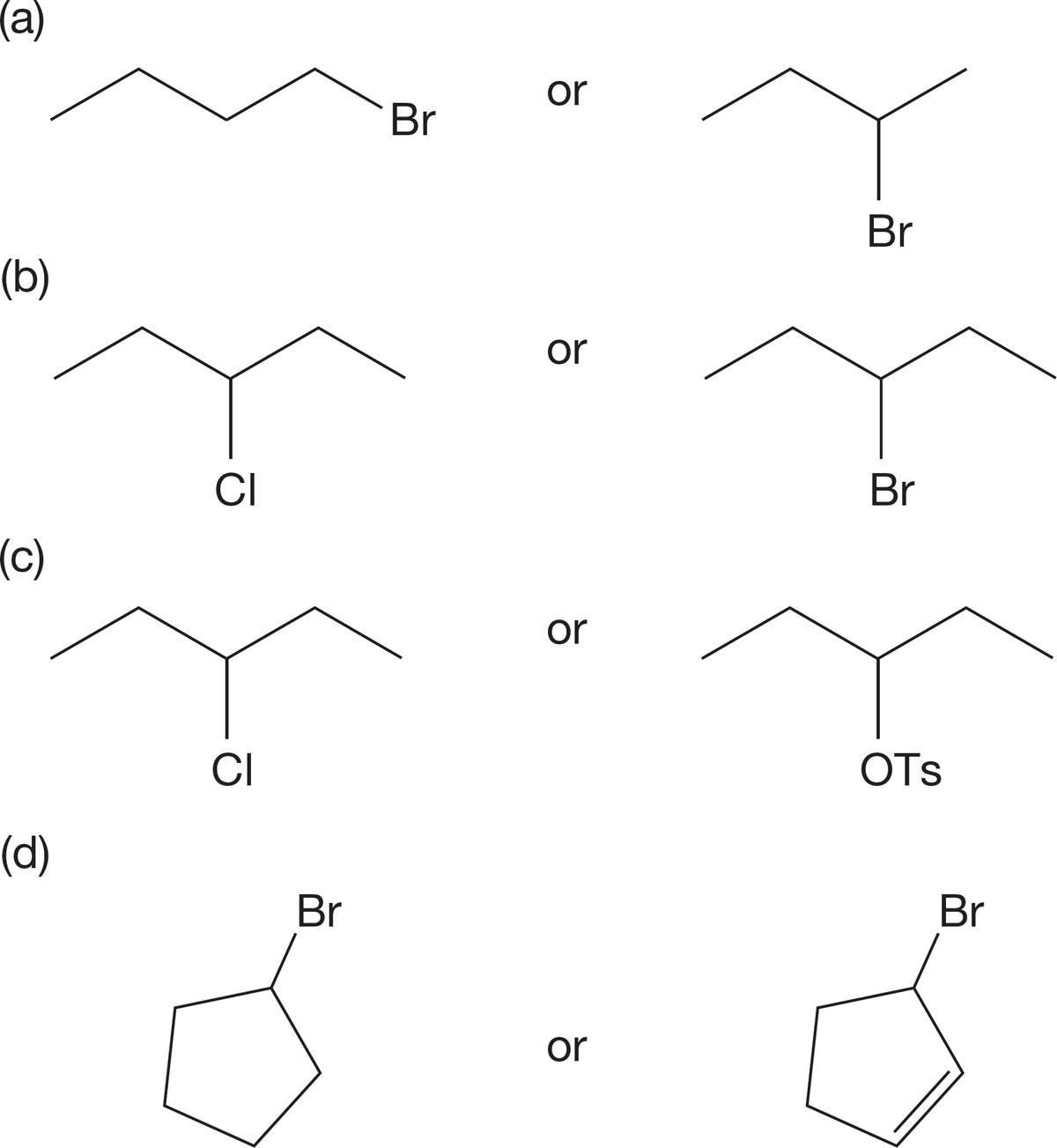
PROBLEM 7.58 Each of the following molecules contains two halogens of different kinds. They are either in different positions in the molecule or are different halogens entirely. In each case, determine which of the two halogens will be the more reactive in the SN2 reaction. Explain.
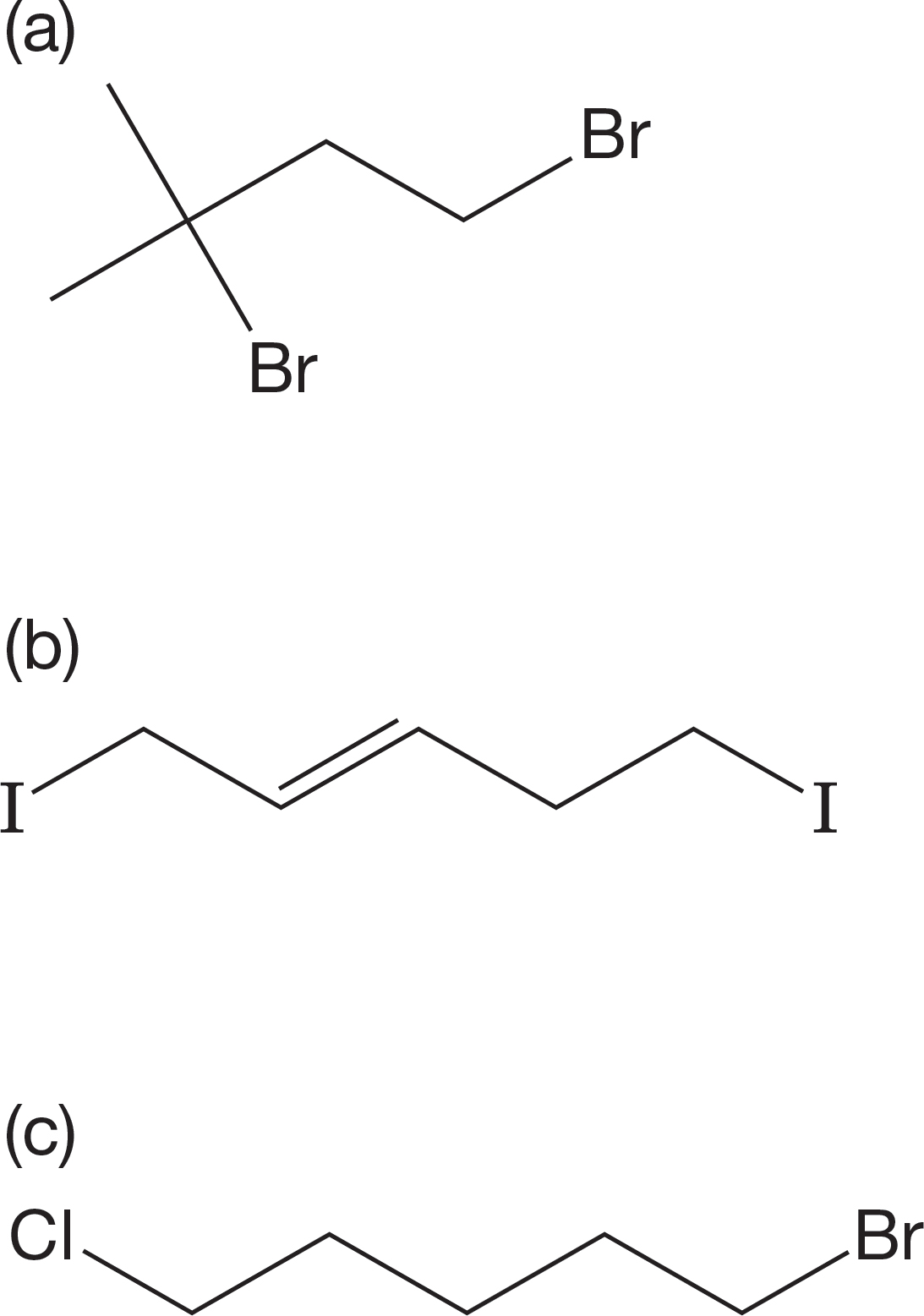
PROBLEM 7.59 Each of the following molecules contains two halogens at different positions in the molecule. In each case, determine which of the two halogens will be the more reactive in the SN1 reaction. Explain.
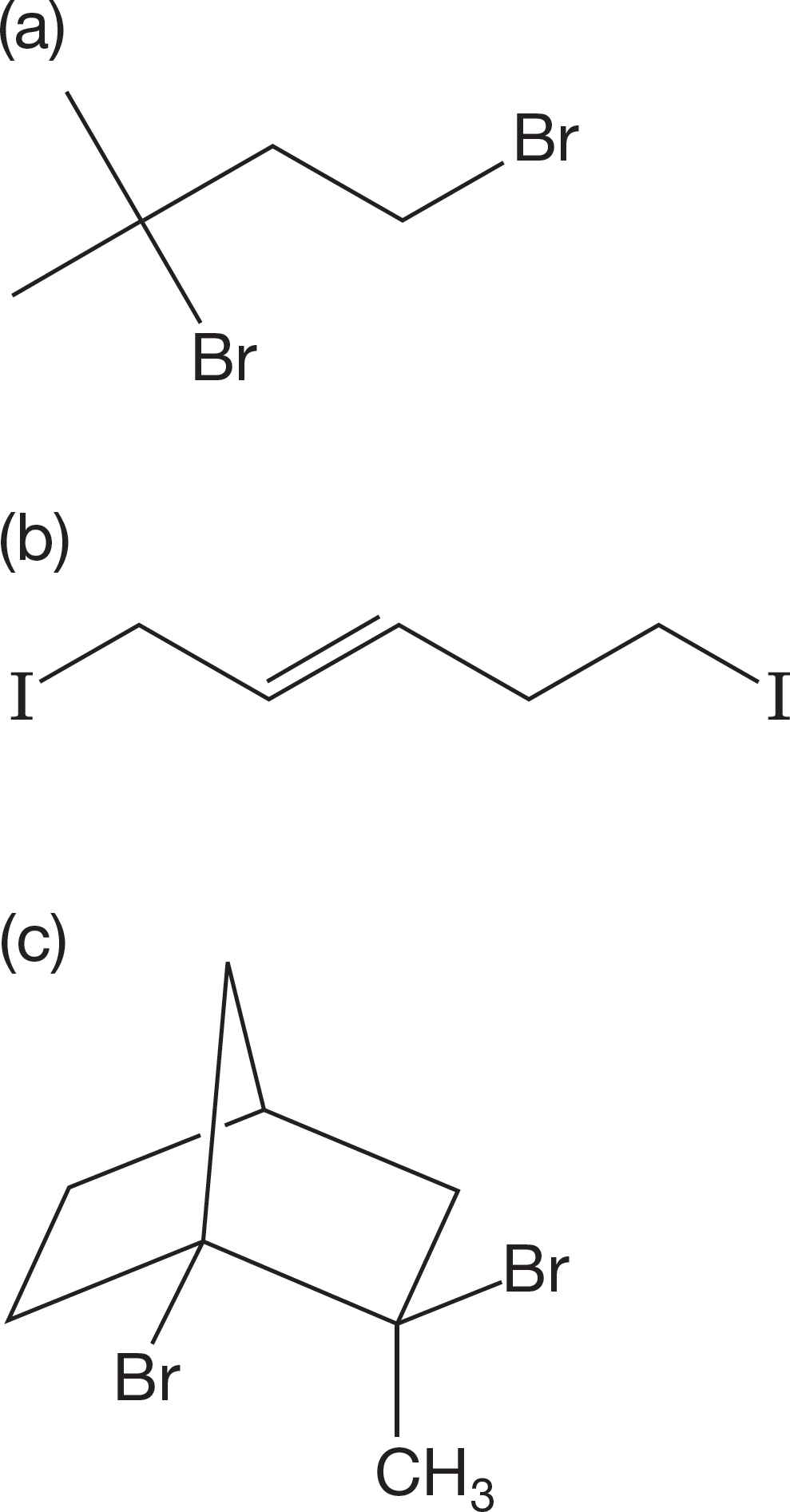
PROBLEM 7.60 You have two bottles containing the two possible diastereomers of the compound shown in the following figure (C8H11IO2). When the salts of these two carboxylic acids are made, only one of them is converted into a new compound, C8H10O2. The other does not react. What is the structure of the new compound, and how does its formation allow you to determine the stereochemistry of the original two compounds? The squiggly bond indicates both stereoisomers.
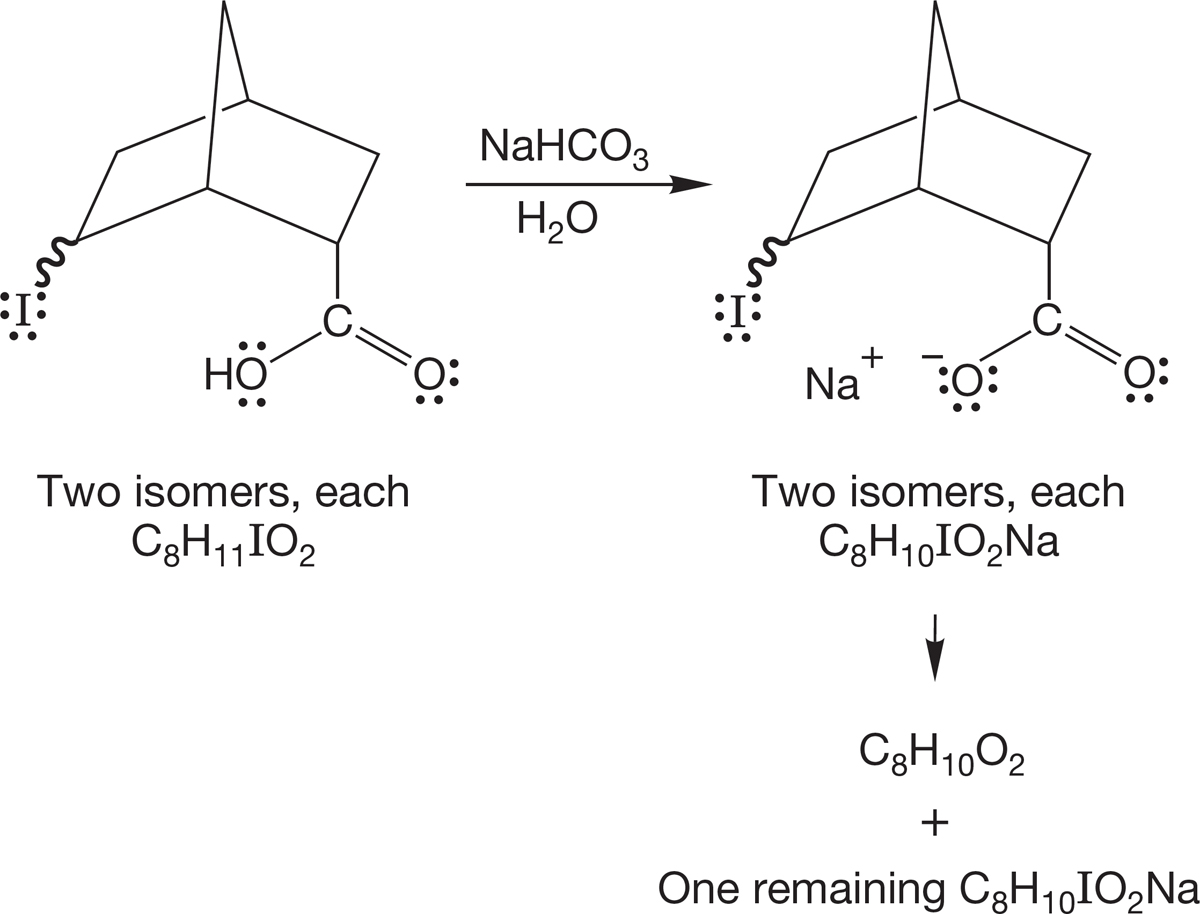
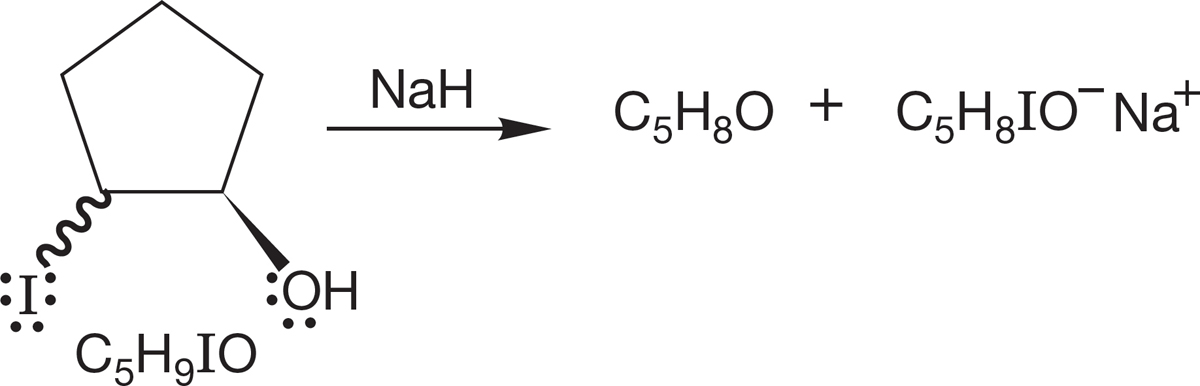
PROBLEM 7.61 You have two bottles containing the two possible diastereomers of the compound shown in the following figure (C5H9IO). When the molecules are treated with a strong base, such as sodium hydride, only one of them is converted into a new compound, C5H8O. What is the new compound, and how does its formation allow you to determine the stereochemistry of the original two compounds?
PROBLEM 7.62 (a) Write the “obvious” arrow formalism for the following transformation. Of course it is going to be wrong; you are being set up here. But don’t mind, it’s all a learning experience.
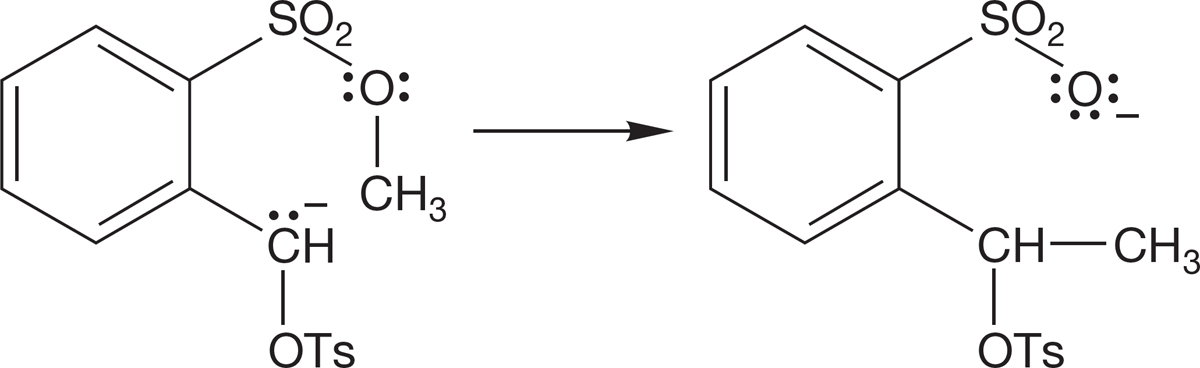
(b) Okay, what you wrote is almost certainly wrong. To find the correct mechanism, you measure the kinetics of this process and discover that the reaction is bimolecular. Now write another arrow formalism.
(c) What’s wrong with the mechanism suggested by the original arrow formalism? Hint: Think about the geometry of the transition state for the SN2 reaction.
PROBLEM 7.63 If you were to add the methoxide base to propane, would propane have a hydrogen acidic enough to react with the base? In other words, would you predict a reaction? See Table 6.6 (p. 246) if you need pKa information.
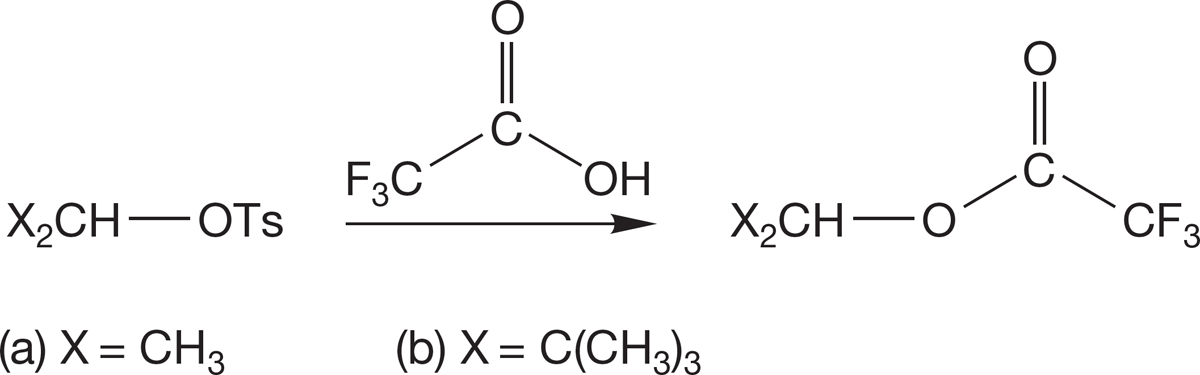
PROBLEM 7.64 The two secondary tosylates undergo solvolysis in trifluoroacetic acid as shown. The second reaction (b) occurs 105 times faster than the first (a). Explain why the second reaction is faster. Construct an energy versus reaction progress diagram comparing the two reactions.
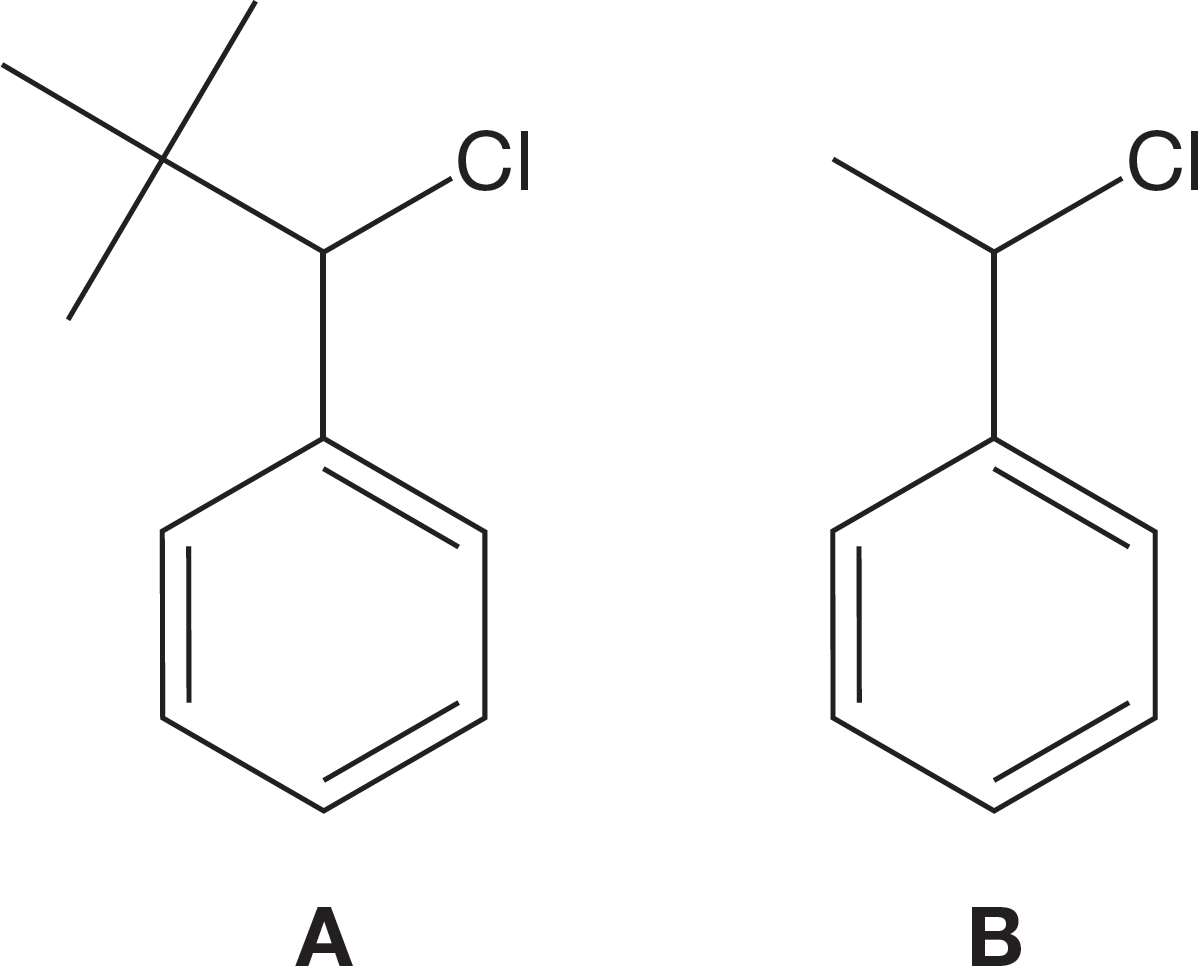
PROBLEM 7.65 Explain why compound A would be slower in the SN1 reaction than compound B. Hint: Draw out each intermediate carbocation and visualize it in three dimensions.
PROBLEM 7.66 Assume you have a bottle of enantiomerically pure (R)-2-butanol and any other reagents you might need. Show the reactions you would use to make enantiomerically pure (S)-2-iodobutane. Show the reactions you would use to make enantiomerically pure (R)-2-iodobutane.
PROBLEM 7.67 In general, vinyl halides do not undergo SN2 reactions. There are at least three very good explanations for this observation. Give two reasons for the lack of reactivity. Can you think of other reasons?
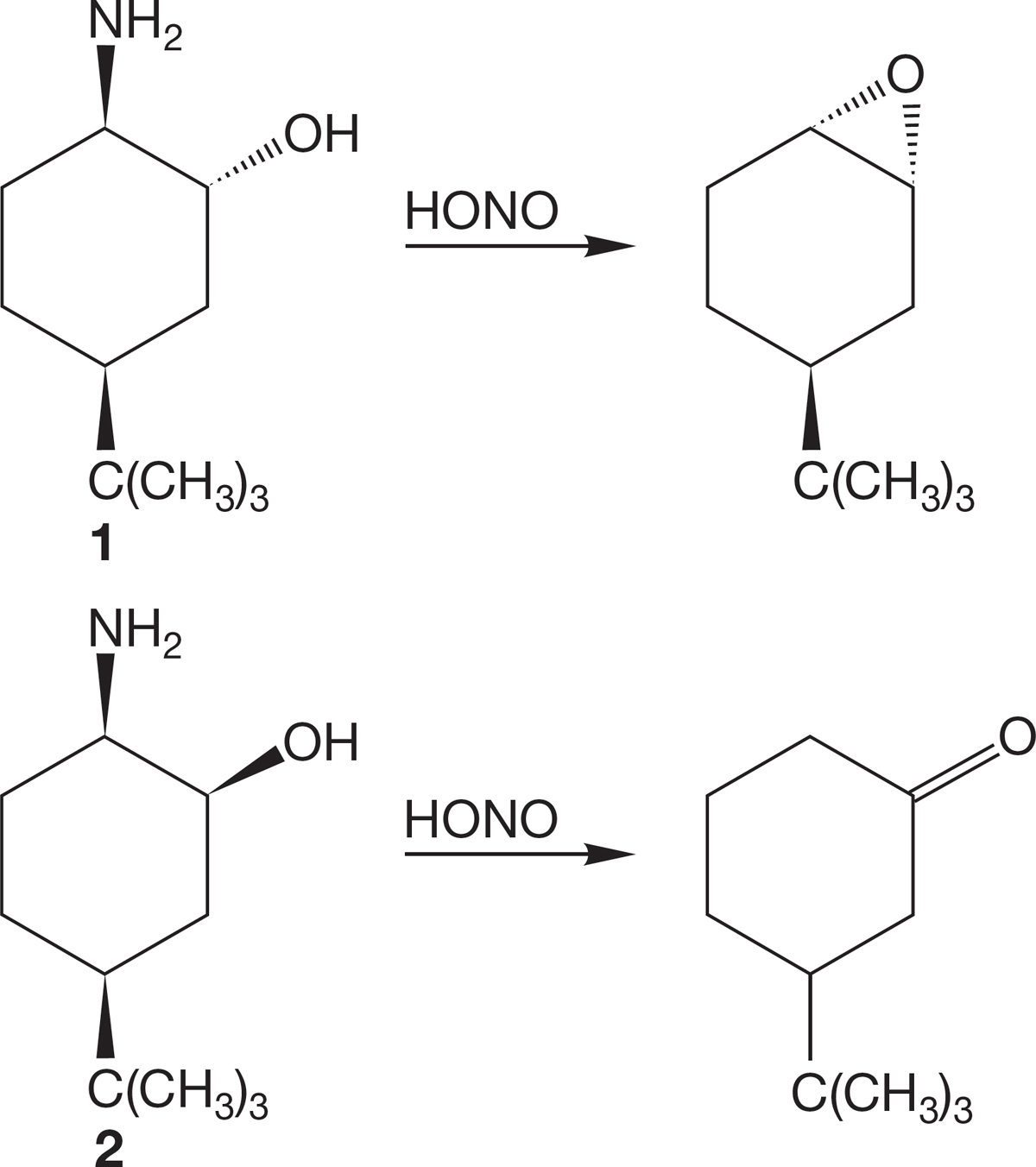
PROBLEM 7.68 Treatment of amines, R―NH2 with nitrous acid, HONO, gives diazonium ions, R―N2+. The N2 is the world’s best leaving group. Rationalize the different products formed on treatment of the stereoisomeric aminocyclohexanols 1 and 2 with nitrous acid.
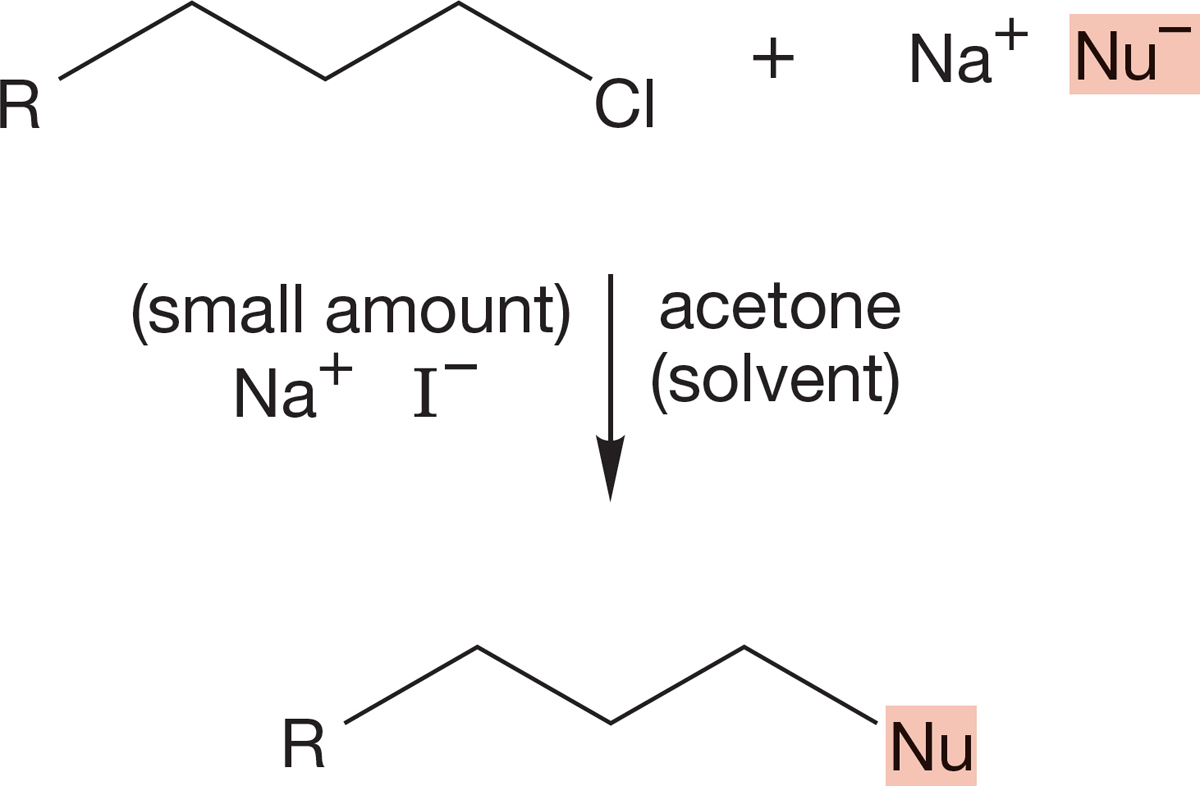
PROBLEM 7.69 A common procedure to increase the rate of an SN2 displacement is to add a catalytic (very small) amount of sodium iodide. Notice that even though the rate increases, the structure of the product is not the iodide. Explain the function of the iodide. How does it act to increase the rate, and why is only a catalytic amount necessary?
Use Organic Reaction Animations (ORA) to answer the following questions:
PROBLEM 7.70 Select the animation titled “Halide formation” in the panel of reactions labeled Semester 2A. Write out the reaction (see Fig. 7.61). How many intermediates are included in the animation? According to the energy diagram shown for the reaction, which step is the fastest?
PROBLEM 7.71 The “Halide formation” reaction has several intermediates formed that aren’t shown in the animation. Predict what they are. It might help to know that one of the final products in the reaction is P(OH)3.
PROBLEM 7.72 After you watch the “Halide formation” reaction, you should be able to describe the mechanism of the second step. What kind of reaction would you call it? Do you suppose a tertiary alcohol goes through the same mechanism?
If your instructor assigns problems in  , log in at smartwork.wwnorton.com.
, log in at smartwork.wwnorton.com.
 CHCH2
CHCH2
 CH
CH CH2
CH2