7.6 The SN2 Reaction in Biochemistry
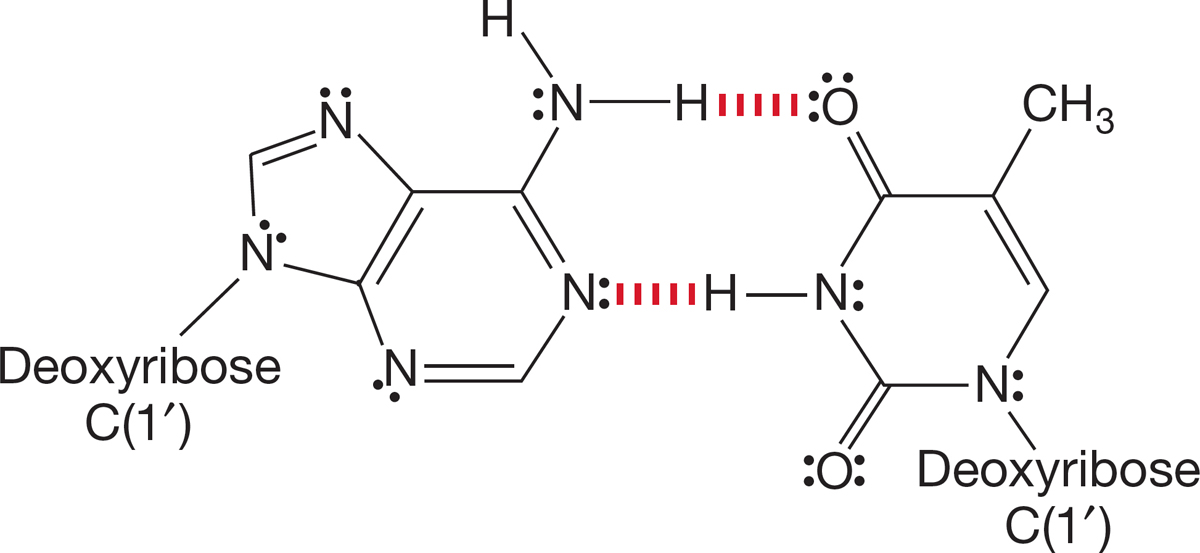
FIGURE 7.67 Replacement of the N―H hydrogens by an alkyl group would hinder—or even eliminate—hydrogen bonding (shown in red). Thus, proper association of the bases would no longer be possible
As mentioned in Section 7.1, your vital biomolecules—DNA, for example—are covered with nucleophiles, each of them ready to undergo the SN2 reaction if presented with a suitable alkylating agent. Once that alkylation takes place, two important changes are induced. First of all, the shape of the molecule changes, and the critical fit, on which proper bioaction depends, is altered. Second, alkylation often removes the possibility for hydrogen bonding, which in turn changes the ability of DNA to associate properly with other molecules. As we will see in Chapter 22, DNA exists as pairs of associated “nitrogenous bases,” and that association is achieved through hydrogen bonding (Fig. 7.67). Alkylation of one of the nucleophilic groups in DNA can disrupt base pairing and lead to destructive mutations.
Bromomethane is one example of a methylating agent that can be found in nature. It is also made industrially on large scales and has been used to sterilize soil. It has a boiling point of 4°C, so it is normally a gas. It can methylate DNA and in high concentrations is carcinogenic. Bromomethane is also an ozone-depleting gas. Thus, industry is replacing CH3Br with alternative chemicals, particularly iodomethane.
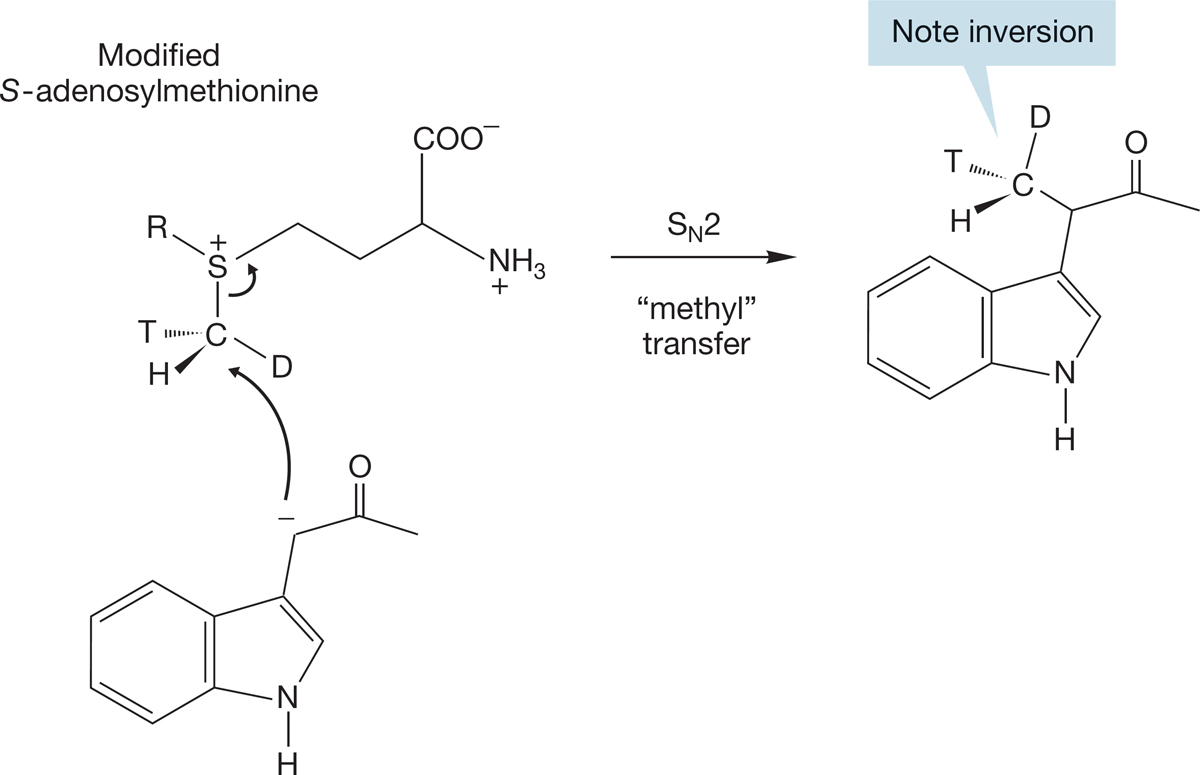
FIGURE 7.68 Methyl transfer involves an SN2 reaction with inversion.
Remember the methyl transfer from S-adenosylmethionine (p. 146)? Heinz Floss (b. 1934) and his co-workers have demonstrated that a similar transfer occurs through an SN2 displacement. Their experiment showed that the transfer takes place with complete inversion of configuration—the hallmark of the SN2 reaction. How can you show inversion in a simple methyl group, CH3? That’s not easy! Floss and his co-workers used two isotopes of hydrogen, deuterium (D), and tritium (T), so that their methyl was not CH3 but CHDT. Use of a single enantiomer revealed the diagnostic inversion in the transfer shown in Figure 7.68. The work involved in synthesizing that single enantiomer and in analyzing the product was prodigious, but these prototypal techniques have been borrowed several times in similar studies of biochemical transformations.
The SN2 reaction is by no means irrelevant to you—your life literally depends on it happening properly, as in the example studied by Floss, and not in the wrong place, as demonstrated by the sometimes lethal mutations induced by alkylating agents.