7.1 Preview
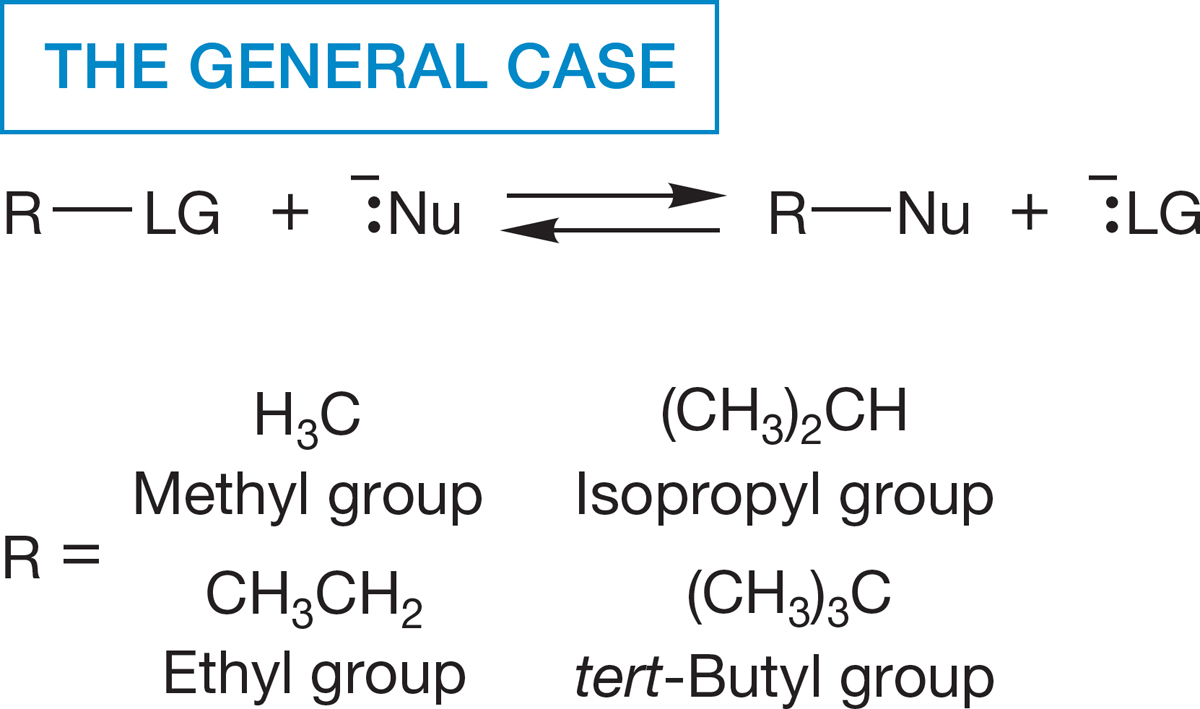
FIGURE 7.1 This generic reaction presents an overall picture of the substitution process. A leaving group, LG, is replaced by a nucleophile, Nu, to give R―Nu. A standard set of alkyl groups that will be used throughout this chapter is presented.
We are about to embark on an extensive study of chemical change. The first six chapters of this book mostly covered various aspects of structure, and still more analysis of structure will appear as we go along. Without this structural underpinning, no serious exploration of chemical reactions is possible. The key to all studies of reactivity is an initial, careful analysis of the structures of the molecules involved. So far, we have been learning parts of the grammar of organic chemistry; now we are about to use that grammar to write some sentences and paragraphs. We begin with a reaction central to most of organic and biological chemistry, the replacement of one group by another (Fig. 7.1).
When one group is replaced by another in a chemical reaction, the result is a substitution reaction. Conversion of one alkyl halide into another is one example of the substitution reaction (Fig. 7.2). This reaction looks simple, and in some ways it is. The only change is the replacement of one halogen by another. Yet as we examine the details of this process, we find ourselves looking deeply into chemical reactivity. This reaction is an excellent prototype that nicely illustrates general techniques used to determine reaction mechanisms.
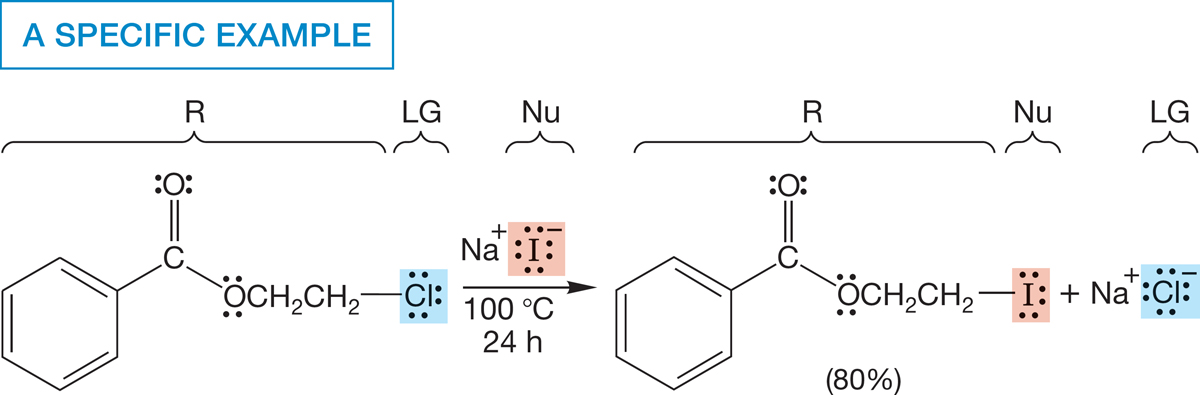
FIGURE 7.2 The replacement of one halogen by another is an example of a substitution reaction.
One reason that we spend so much time on the substitution reaction is that it is so general; what we learn about it and from it is widely applicable. Not only will we apply what we learn here to many other chemical reactions, but also we will recognize that substitution reactions have vast practical consequences. Substitution reactions, commonly called displacement reactions, are at the heart of many industrial processes and are central to the mechanisms of many biological reactions, including some forms of carcinogenesis, the cancer-causing activity of many molecules. Biomolecules are bristling with substituting agents. It seems clear that when some groups on our deoxyribonucleic acid (DNA; see Chapter 22) are modified in a substitution reaction, tumor production is initiated or facilitated. Beware of substituting agents.
This chapter is long, important, and possibly challenging. In it, we cover two of the five building block reactions, the SN1 and SN2 substitution reactions. We have already encountered another fundamental reaction, the addition of HX (see Chapter 3), and it will reappear shortly, in Chapter 10. From now on, you really cannot memorize your way through the material; you must try to generalize. Understanding concepts and applying them in new situations is the route to success. This chapter contains lots of problems, and it is important to try to work through them as you go along. We know this warning is repetitious, and we don’t want to insult your intelligence, but it would be even worse not to alert you to the change in the course that takes place now or not to suggest ways of getting through this new material successfully. You cannot simply read this material and hope to get it all. You must work with the text and the various people in your course, the professor and the teaching assistants, in an interactive way. The purpose of the problems is to help you do this. When you hit a snag or can’t do a problem, find someone who can and get the answer. The answer is more than a series of equations or structures and arrows; it also involves finding the right approach to the problem. There is much technique to problem solving in organic chemistry; it can be learned, and it gets much easier with practice. Remember: Organic chemistry must be read with a pencil in your hand.
ESSENTIAL SKILLS AND DETAILS
1. We have seen the curved arrow formalism before, as early as Chapter 1, but in this chapter it becomes more important than ever. If you are at all uncertain of your ability to “push” electrons with arrows, now is definitely the time to solidify this skill. In the arrow formalism convention, arrows flow from electrons. But be careful about violating the rules of valence; arrows either displace another pair of electrons or fill a “hole”—an empty orbital.
2. It is really important to be able to generalize the definition of Lewis bases and Lewis acids. All electron pair donors are Lewis bases (nucleophiles), and nearly any pair of electrons can act as a Lewis base under some conditions. Similarly, there are many very different appearing Lewis acids (electrophiles). Section 7.2 will review these ideas.
3. The two fundamental reactions we study in this chapter, the SN2 and SN1 substitutions, are affected by the reaction conditions as well as by the structures of the molecules themselves. These two reactions sometimes compete with each other, and it is important to be able to select conditions and structures that favor one reaction over the other. You will not always be able to find conditions that give specificity, but you will usually be able to choose conditions that give selectivity, which will allow you to predict products in substitution reactions.
4. At the end of this chapter, we will sum up what we know about making molecules—synthesis. ALWAYS attack problems in synthesis by doing them backward. Do not attempt to see forward from starting material to product. In a multistep synthesis, this approach is often fatal. The way to do it is to look at the ultimate product and ask yourself what molecule might lead to it in a single step. That technique will usually produce one or more candidates. Then apply the same question to those candidates, and before you know it, you will be at the indicated starting material. This technique works and can make easy what is otherwise a tough, vexing task.
5. Alcohols are important starting materials for synthesis—they are inexpensive and easily available. Alcohols can be made even more useful by learning how to manipulate the OH group to make it more reactive—to make it a better “leaving group.” Several ways to do that are outlined in Section 7.5g.
6. Keeping track of your growing catalog of useful synthetic reactions is not easy—it is a subject that grows rapidly. A set of file cards on which you collect ways of making various types of molecules is valuable. See p. 323 for an outline of how to make this catalog.