
CHAPTER THREE 3
Energy, Catalysis, and Biosynthesis
One property above all makes living things seem almost miraculously different from nonliving matter: they create and maintain order in a universe that is tending always toward greater disorder. To accomplish this remarkable feat, the cells in a living organism must continuously carry out a never-ending stream of chemical reactions to maintain their structure, meet their metabolic needs, and stave off unrelenting chemical decay. In these reactions, small organic molecules—amino acids, sugars, nucleotides, and lipids—can be taken apart or modified to supply the many other small molecules that the cell requires. These molecules can also be used to construct an enormously diverse range of large molecules, including the proteins, nucleic acids, and other macromolecules that constitute most of the mass of living systems and endow them with their distinctive properties.
Each cell can be viewed as a tiny chemical factory, performing many millions of reactions every second. This incessant activity requires both a source of atoms in the form of food molecules and a source of energy. Both the atoms and the energy must come, ultimately, from the nonliving environment. In this chapter, we discuss why cells require energy, and how they use energy and atoms from their environment to create and maintain the molecular order that makes life possible.
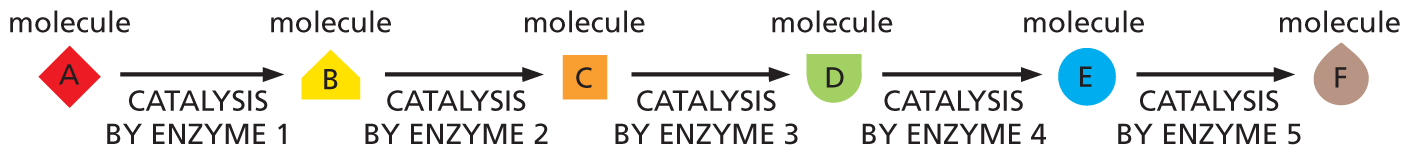
Most of the chemical reactions that cells perform would normally occur only at temperatures that are much higher than those inside a cell. Each reaction therefore requires a major boost in chemical reactivity to enable it to proceed rapidly within the cell. This boost is provided by a large set of specialized proteins called enzymes, each of which accelerates, or catalyzes, just one of the many possible reactions that a particular molecule could in principle undergo. These enzyme-catalyzed reactions are usually connected in series, so that the product of one reaction becomes the starting material for the next (Figure 3–1). The long, linear reaction pathways that result are in turn linked to one another, forming a complex web of interconnected reactions. Rather than being an inconvenience, the necessity for catalysis is critical for life, as it allows the cell to precisely control its metabolism—the sum total of all the chemical reactions it needs to carry out to survive, grow, and reproduce.
More information
An illustration shows the phases in an enzyme-catalyzed reaction. Molecule A undergoes catalysis by Enzyme 1 to produce molecule B, which undergoes catalysis by Enzyme 2 to produce molecule C. Molecule C undergoes catalysis by Enzyme 3 to produce molecule D which further undergoes catalysis by Enzyme 4 to produce molecule E. Molecule E undergoes catalysis by Enzyme 5 to produce molecule F.
Figure 3–1 A series of enzyme-catalyzed reactions forms a linked pathway. Each chemical reaction is catalyzed by a distinct enzyme. Together, this set of enzymes, acting in series, converts molecule A to molecule F.
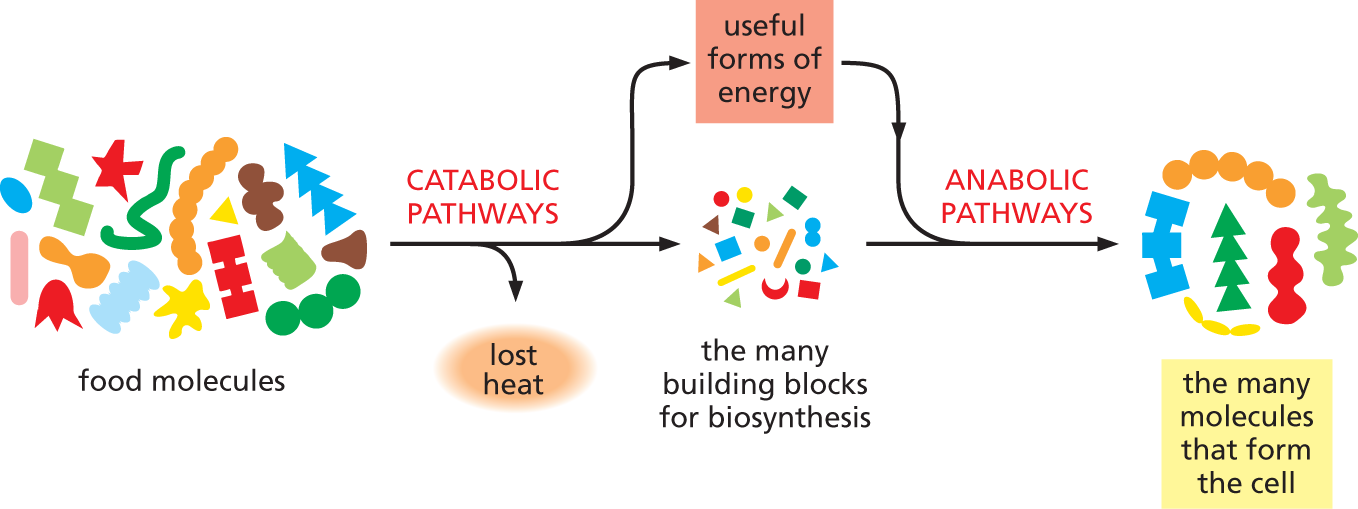
There are two opposing streams of chemical reactions in cells: the catabolic pathways and the anabolic pathways. The catabolic pathways (catabolism) break down foodstuffs and other materials—including cell components that have become damaged—into smaller molecules, thereby generating both a useful form of energy for the cell and small molecules that the cell can use as building blocks. The anabolic, or biosynthetic, pathways (anabolism) use the energy harnessed by catabolism to drive the synthesis of the many molecules that form the cell. Together, these two sets of reactions constitute the metabolism of the cell (Figure 3–2).
More information
An illustration shows catabolic and anabolic pathways that constitute a cell’s metabolism. Food molecules are converted into useful forms of energy by catabolic pathways and heat is lost in the process; food molecules are also broken down to form the many building blocks for biosynthesis. The energy obtained runs the anabolic pathways which synthesize the many molecules that form the cell.
Figure 3–2 Catabolic and anabolic pathways together constitute the cell’s metabolism. During catabolism, a major portion of the energy stored in the chemical bonds of food molecules is dissipated as heat. But some of this energy is converted to the useful forms of energy that are needed to drive the synthesis of new molecules in anabolic pathways, as indicated.
Most of the details regarding the reactions that comprise cell metabolism are part of the subject matter of biochemistry, and they need not concern us here. But the general principles by which cells obtain energy from their environment and use it to create order are central to cell biology. We therefore begin this chapter by explaining why a constant input of energy is needed to sustain life. We then discuss how enzymes catalyze the reactions that produce biological order. Finally, we describe the molecules inside cells that carry the energy that makes life possible.
Glossary
- metabolism
- The sum total of the chemical reactions that take place in the cells of a living organism.
- catabolism
- Set of enzyme-catalyzed reactions by which complex molecules are degraded to simpler ones with release of energy; intermediates in these reactions are sometimes called catabolites.
- anabolism
- Set of metabolic pathways by which large molecules are made from smaller ones.