2.14 Nuclear Magnetic Resonance Spectra of Alkanes
Spectroscopy is the study of molecules through the investigation of their interaction with electromagnetic radiation. There are many kinds of spectroscopy (as we shall see in Chapter 9). One version is called nuclear magnetic resonance (NMR) spectroscopy and is particularly valuable, both in chemistry as a device for determining molecular structure and in medicine as an imaging tool. You have heard of this form of spectroscopy before if you have ever read an article about magnetic resonance imaging (MRI). NMR and MRI are the same process, but MRI avoids the word nuclear, which has some unfavorable associations for the public.
NMR spectroscopy is an essential tool for analysis of molecules. For example, it tells us about the symmetry of a molecule. The topic of symmetry is essential to the material we will learn in Chapter 4, and it is a critical topic ever after that. By the end of the course you will gain an understanding of molecular symmetry that will change your view of life. And it is NMR that verifies our assignment of symmetry to a molecule. So we need this tool and it makes sense to introduce it now, where we are just starting to see issues of symmetry.
Another reason for getting you started on NMR at this early point has to do with the organic laboratory course that many students take concurrently with their organic lecture course. If you are enrolled in the lab, you probably will have exposure to NMR spectroscopy in that setting. Having the topic introduced now will help strengthen your use of this tool. If you don’t have the associated organic lab, then this introduction will help prepare you for the full topic in Chapter 9. Either way, we believe it is a valuable tool that should be used to verify molecular symmetry. Read on!
Although we won’t go into much detail yet, this early introduction to nuclear magnetic resonance does allow us to address the critical question of difference. When are two atoms the same and when are they different?
Like electrons, nuclei of many atoms have a property called spin. A nonzero nuclear spin is necessary for a nucleus to be NMR-active and thus detectable by an NMR spectrometer. The 13C and 1H nuclei each have spins of ±1/2, just like the electron. Although 13C is present in only 1.1% abundance in ordinary carbon, which is mostly 12C, that small amount can be detected.
Like the electron, the 13C and 1H nuclei can be thought of as spinning in one of two directions. In the presence of a strong magnetic field, those two spin states differ in energy, but by only a tiny amount. Nonetheless, transitions between the two states can be detected by NMR spectrometers tuned to the proper frequency. We’ll have more to say about those spectrometers and those transitions in Chapter 9, but there is really not much more to it than that. So we can see a signal whenever a transition between the lower-energy and the higher-energy nuclear spin states is induced. So what? It would seem that we have simply built a (very expensive) machine to detect carbon or hydrogen in a molecule, and it would hardly be surprising to find such atoms in organic molecules!
The critical point is that every different carbon (or hydrogen) in a molecule—every such atom in a different environment, no matter how slightly different—gives a signal that is different from that of the other carbons (or hydrogens) in the molecule. The NMR spectrometer can “count” the number of different carbons or hydrogens in a molecule by counting the number of signals. That ability can be enormously useful in structure determination, and NMR spectroscopy is very often used by “the pros” of structure determination in exactly that way. The array of signals is called the NMR spectrum of the molecule.
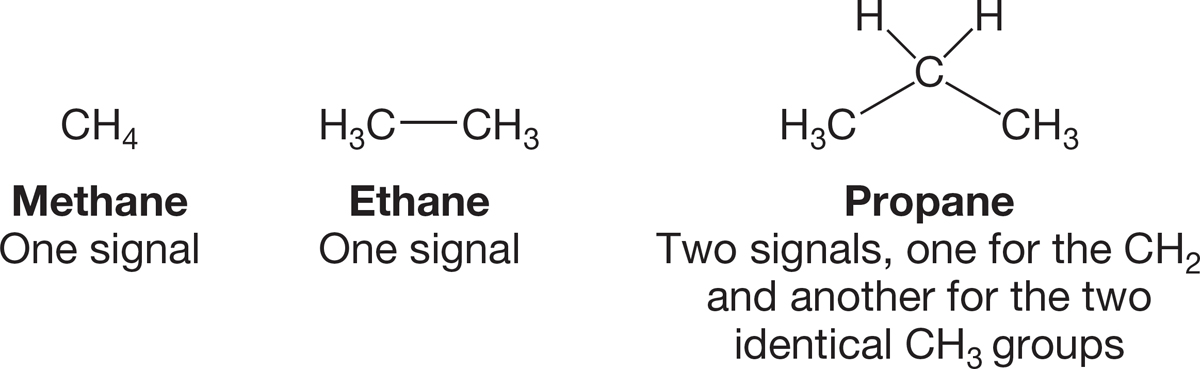
FIGURE 2.56 Carbon-13 NMR signals for three alkanes.
Let’s use Figure 2.56 to look at a few examples. How many signals will a 13C NMR spectrometer “see” for methane? One, of course, because there is only one carbon. How about ethane? There are two carbons here, but they are in identical environments and thus equivalent: one signal again. How about propane? Three carbons are here, two methyl groups and a single methylene. Surely, we must observe a different signal for that CH2 than for the CH3 groups. And we do—there are two signals in the 13C NMR spectrum for propane: one for the CH2 and another for the two equivalent CH3 groups.
Counting the number of signals is trivial if (and only if) we can discern whether the molecular environments of the carbons are the same or not. Here is a slightly harder example. The molecule tetrahydrofuran produces two 13C signals (Fig. 2.57). It is tempting to say that because there are only methylene (CH2) groups in this molecule, there should only be one signal. But those methylenes are not all the same. Two of them are adjacent to the oxygen, and two are not. So there are two signals, one for each set of two different methylenes.
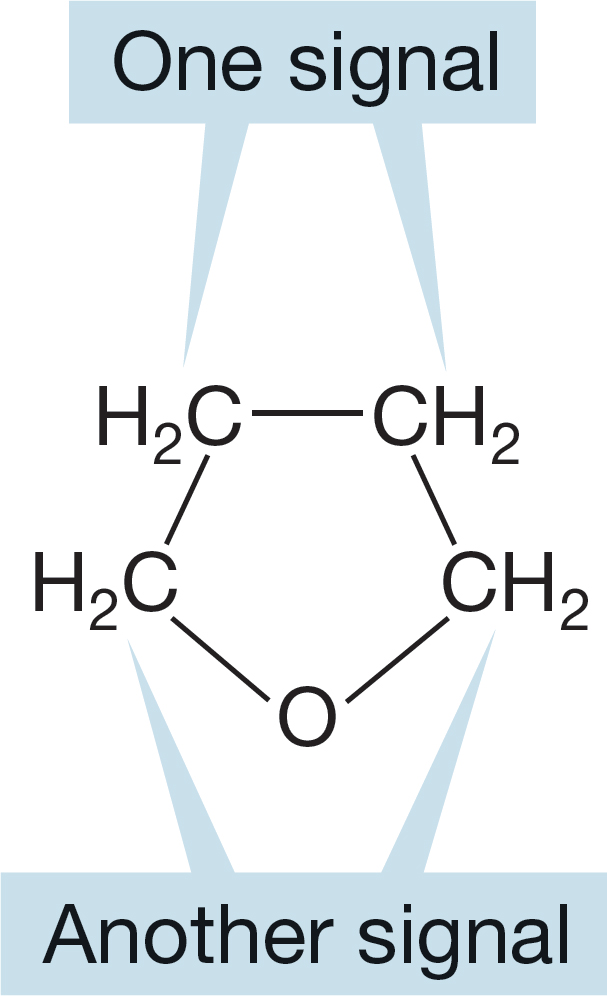
FIGURE 2.57 Tetrahydrofuran (THF) has two different methylene groups.
We will pick these points up again as we discuss the various structural types in the next few chapters, but for the moment, work through the following Problem Solving box and then try a few problems on your own.
PROBLEM SOLVING
Nearly everyone has initial problems with seeing differences in the atoms making up any molecule. Why, for example, are there three different methylene (CH2) groups in heptane? Maybe it is intuitively obvious (maybe!) that the central methylene group is different from the others, but almost everyone worries about the other methylene groups.
The best technique to use when you are in doubt is to list all the groups to which the carbons in question are attached. And be very detailed in making that list—do not just look at nearest neighbors. Here is an example using heptane:
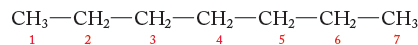
Carbon 1 methyl: Attached to C―C―C―C―C―C, same as carbon 7 methyl
Carbon 2 methylene: Attached to C on one side and to C―C―C―C―C on the other, same as carbon 6 methylene
Carbon 3 methylene: Attached to C―C and to C―C―C―C, same as carbon 5 methylene
Carbon 4 methylene: Attached to C―C―C and to C―C―C
Carbon 5 methylene: Attached to C―C―C―C and to C―C, same as carbon 3 methylene
Carbon 6 methylene: Attached to C―C―C―C―C and to C, same as carbon 2 methylene
Carbon 7 methyl: Attached to C―C―C―C―C―C, same as carbon 1 methyl
Therefore, heptane will have four signals in its 13C NMR spectrum: one signal for the equivalent methyl groups (carbons 1 and 7), one signal for the methylenes of carbons 2 and 6, one signal for the methylenes of carbons 3 and 5, and one signal for the carbon 4 methylene.
PROBLEM 2.34 How many 13C signals will we see in an NMR spectrum of the molecules in Figures 2.46–2.48?
PROBLEM 2.35 How many 13C signals will an NMR spectrometer detect in the molecules of Figure 2.50?
PROBLEM 2.36 How many 13C signals will an NMR spectrometer detect in the molecules of Figure 2.53?
Hydrogen NMR (1H NMR) spectroscopy is similar to 13C NMR spectroscopy. The areas of the signals we observe in 1H NMR spectroscopy are in the ratio of the numbers of different hydrogens giving rise to those signals. For example, the signal recorded for six hydrogens of one kind will give a signal three times as big as a signal for two hydrogens, and so on. These ratios from the signals are more reliably determined in 1H NMR than they are in 13C NMR. There are other very useful complications introduced by the abundance of the NMR active isotope (1H) in organic molecules, but we can leave them for Chapter 9. We will use some of the molecules introduced in this chapter to work through a bit of hydrogen NMR. We’ll find a few more subtleties, but the overall picture will not be very different from what we have seen for 13C NMR spectroscopy.
PROBLEM 2.37 What will be the ratios of the signals in the 1H NMR spectra for propane (Fig. 2.56) and THF (Fig. 2.57)?
PROBLEM 2.38 How many signals would be seen in the 1H NMR spectrum for heptane (Fig. 2.45)? What will the relative sizes of these signals be?
PROBLEM 2.39 How many signals would be seen in the 1H NMR spectrum for cis-1,2-dimethylcyclopropane (Fig. 2.52)? Caution: This question is a bit tricky—look carefully at a model or use the Web site to see cis-1,2-dimethylcyclopropane in three dimensions.