2.12 Cycloalkanes
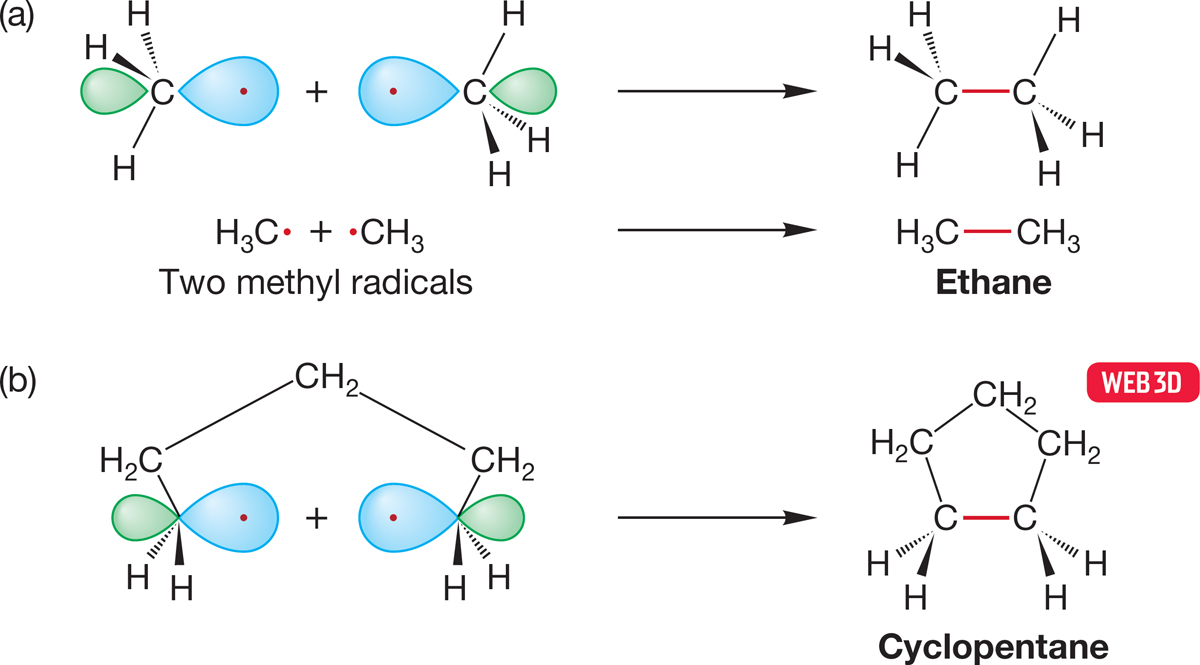
FIGURE 2.49 (a) The formation of ethane through the overlap of two singly occupied sp3 hybrid orbitals. (b) The closely related formation of a ring compound, cyclopentane, through the overlap of two singly occupied sp3 hybrid orbitals.
All the hydrocarbons we have met so far have the molecular formula CnH2n+2. Because these molecules are linear or branched chains, we refer to them as either noncyclic or acyclic alkanes. Molecules of this formula are also called saturated hydrocarbons, which means that the molecule has a maximum number of hydrogens for a given number of carbons. There is a class of closely related molecules that shares most chemical properties with the noncyclic alkanes but not the general formula. These molecules have the composition CnH2n and are the cycloalkane ring compounds mentioned briefly in Section 2.1. Cycloalkanes with this formula are also saturated. Molecules that have the formula CnH2n but have no rings are called unsaturated hydrocarbons. Unsaturated compounds have carbon–carbon double (or triple) bonds and will be discussed in Chapter 3.
What might the bonding in cyclic molecules be? As you might guess, the chemical properties of cycloalkanes resemble very closely those of the noncyclic alkanes. There is no essential difference between the process used to consider ethane formed from two methyl radicals (Fig. 2.49a) and this construction of a ring compound (Fig. 2.49b). Nor would we expect to see big differences in chemical properties because the bonding in pentane is not significantly different from that in cyclopentane.
Cycloalkanes are named by attaching the prefix cyclo- to the name of the parent hydrocarbon (Table 2.6).
TABLE 2.6 Some Cyclic Alkanes (Cycloalkanes)
Name |
Formula |
mp (°C) |
bp (°C, 760 mmHg) |
Cyclopropane |
(CH2)3 |
−127.6 |
−32.7 |
Cyclobutane |
(CH2)4 |
−90 |
12 |
Cyclopentane |
(CH2)5 |
−93.9 |
49.2 |
Cyclohexane |
(CH2)6 |
6.5 |
80.7 |
Cycloheptane |
(CH2)7 |
−12 |
118.5 |
Cyclooctane |
(CH2)8 |
14.3 |
148–149 (749 mmHg) |
mp, melting point; bp, boiling point.
Monosubstituted cycloalkanes require no numbers for naming. In polysubstituted ring compounds, substituents are assigned numbered positions, and the ring atoms are numbered so that the lowest possible numbers are used. Multiple substituents are named in alphabetical order in the prefix (Fig. 2.50). Prefix modifiers (di-, tri-, etc.) are dealt with as shown in Table 2.5.
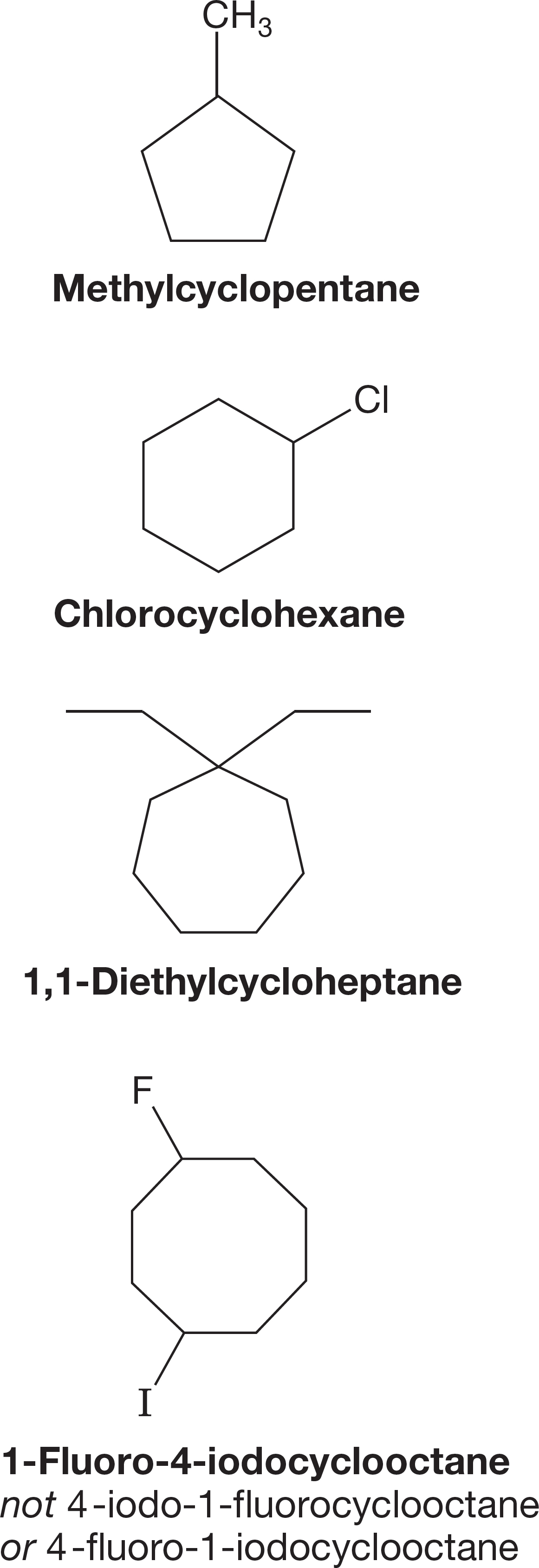
FIGURE 2.50 Cycloalkanes are named and numbered in a fashion similar to the system for acyclic alkanes.
One important new structural feature appears in cycloalkanes. There is only a single methylcyclopropane, which is why there is no number used in naming it. But there are two isomers of 1,2-dimethylcyclopropane. As shown in Figure 2.51, there is no “sidedness” to methylcyclopropane. The molecule with the methyl group “up” can be transformed into the molecule with the methyl group “down” by rotating the molecule 180° about the axis shown in Figure 2.51, in other words, by simply turning the molecule over.
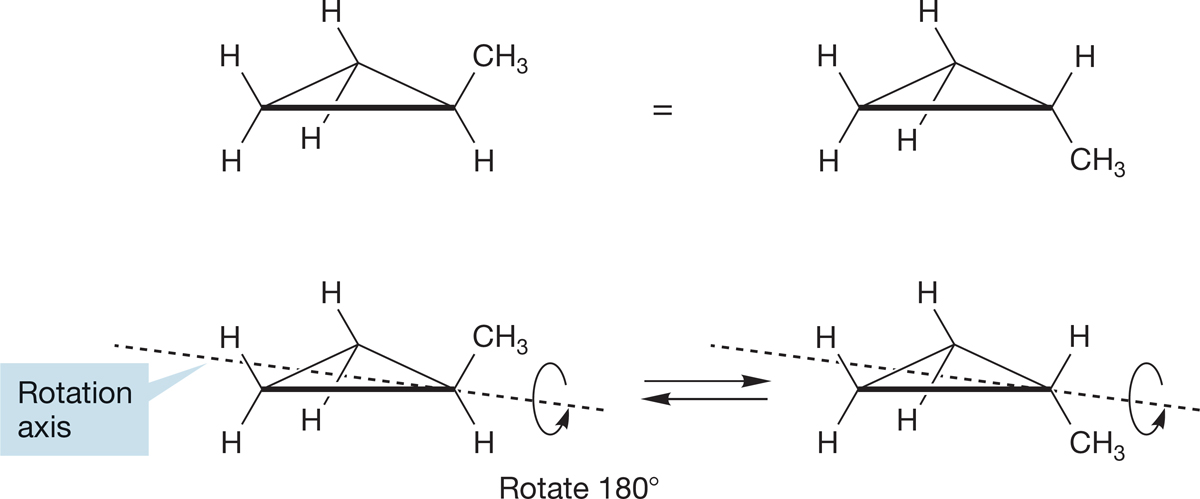
FIGURE 2.51 There is only one isomer of methylcyclopropane. The thicker line in these drawings is used to show the side of the cyclopropane nearest to you.
However, no number of translational or rotational operations can change the 1,2-dimethylcyclopropane with both methyl groups on the same side of the ring, called cis, into the 1,2-dimethylcyclopropane with the methyl groups on opposite sides, called trans (Fig. 2.52). The two molecules are certainly different from each other. The use of models is absolutely mandatory at this point. Make models of these two compounds and convince yourself that nothing short of breaking carbon–carbon bonds will allow you to turn cis-1,2-dimethylcyclopropane into the isomeric trans-1,2-dimethylcyclopropane.
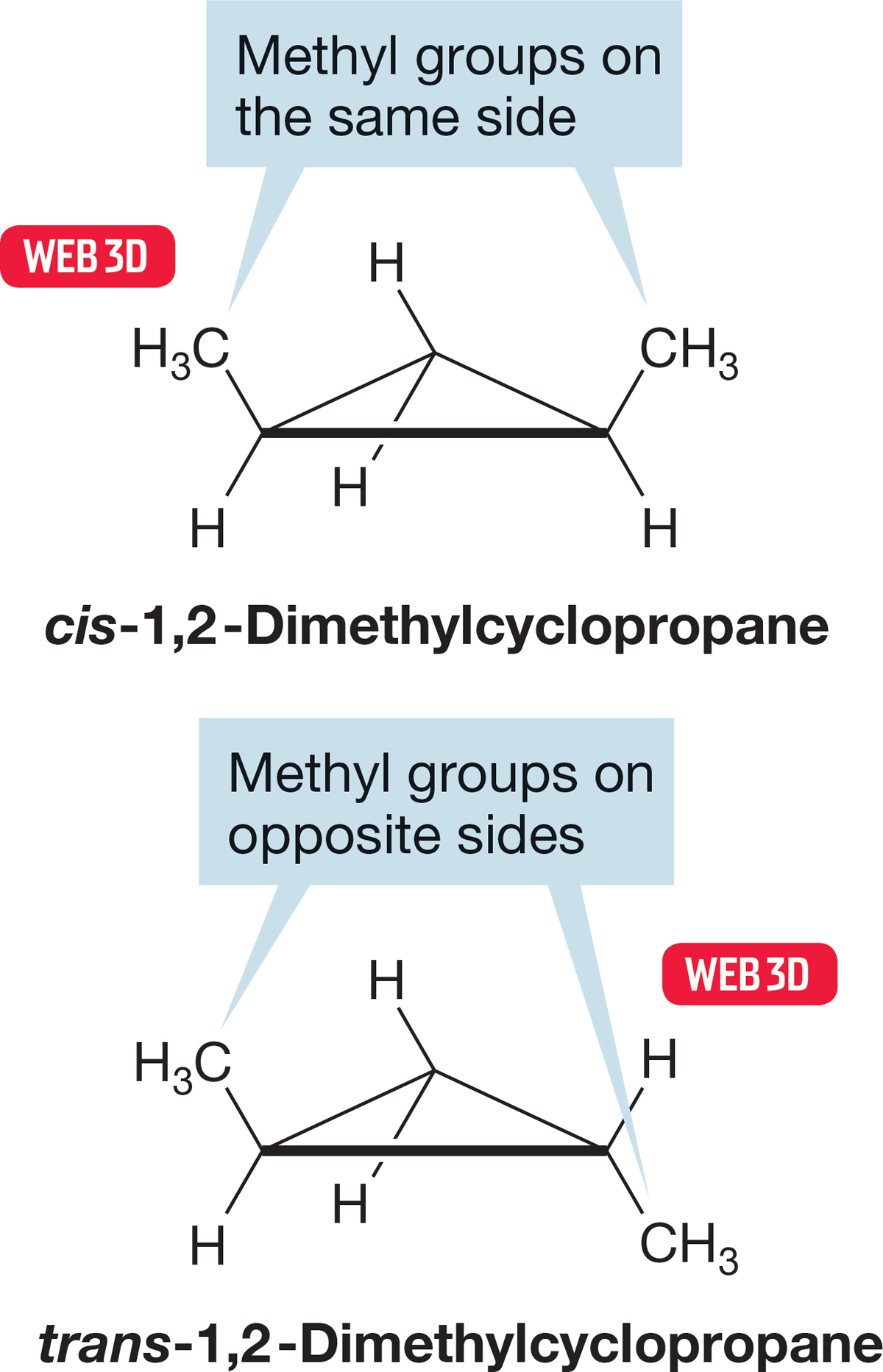
FIGURE 2.52 Two isomers of 1,2-dimethylcyclopropane.
PROBLEM 2.29 1,2-Dimethylcyclopropane is even more complicated than just described, as you will see in Chapter 4, because there are two isomers of trans-1,2-dimethylcyclopropane! Use your models to construct the mirror image of the trans-1,2-dimethylcyclopropane you just made and see if it is identical to your first molecule.
Cyclic compounds are extremely common. Both small and large varieties are found in nature, and many kinds of exotic cyclic molecules not yet found outside the laboratory have been made by chemists. Moreover, cyclic molecules can be combined in several ways to form polycyclic molecules. Here is an opportunity for you to think ahead. How might two rings be attached to each other? Some ways are obvious, but others require some thought. We will work through this topic as a series of four problems, two worked in the chapter, two not. Several different structural types can be created from two rings. These problems lead you through them.
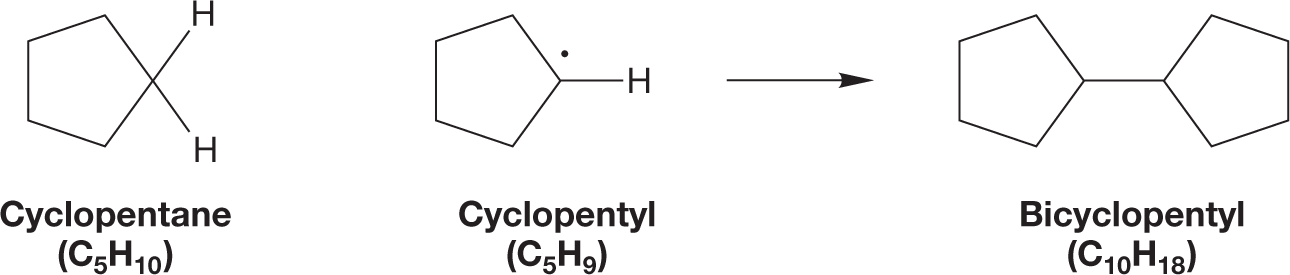
WORKED PROBLEM 2.30 Draw a molecule that has the formula C10H18 and is composed of two five-membered rings.
ANSWER Two five-membered rings can be joined in a very simple way to make bicyclopentyl. There is no real difference between this process and the formation of ethane from two methyl radicals (p. 72).
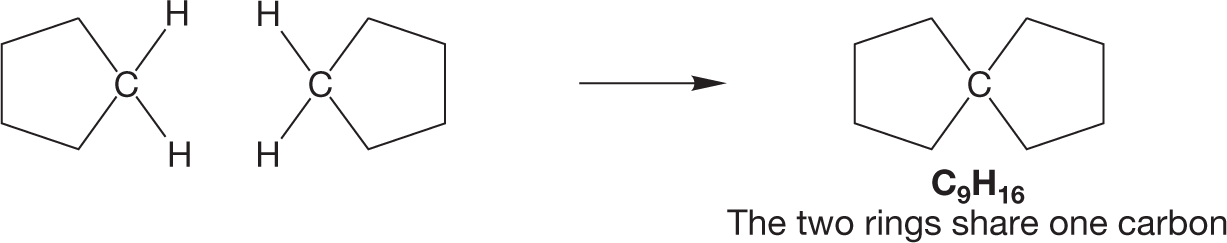
WORKED PROBLEM 2.31 Another molecule containing two five-membered rings has the formula C9H16. Clearly, we are not dealing with a simple combination of two cyclopentanes here because we are short one carbon—it’s C9, not C10, as in bicyclopentyl. The two rings must share one carbon somehow. Draw this compound.
ANSWER The way to have two rings sharing a carbon is to let one carbon be part of both rings:
PROBLEM 2.32 Two five-membered rings can share more than one carbon. Two similar molecules that are structured this way both have the formula C8H14. Draw them. Hint: Focus on the two shared carbons and the hydrogens attached to them. (Make a model.) That’s all the help you get here. Use Problem 2.31 as a guide.
PROBLEM 2.33 Finally, and most difficult, there is a molecule, still constructed from five-membered rings, that has the formula C7H12. In this molecule, the two five-membered rings must share three carbons. Draw this molecule.
Polycyclic compounds can be exceedingly complex. Indeed, much of the fascination that organic chemistry holds for some people is captured nicely by the beautifully architectural structures of these compounds. Figure 2.53 shows three examples of cyclic molecules. Aflatoxin B1 and progesterone are found in nature, and we will refer to such compounds as natural products. The compound [1.1.1]propellane (we’ll learn the numbering system for bicylic compounds in Chapter 5) is not yet found outside the laboratory. Aflatoxin B1 is a highly toxic fungal metabolite. Progesterone is one of a class of molecules called steroids; it has an antiovulatory effect if taken during the middle days of the menstrual cycle.
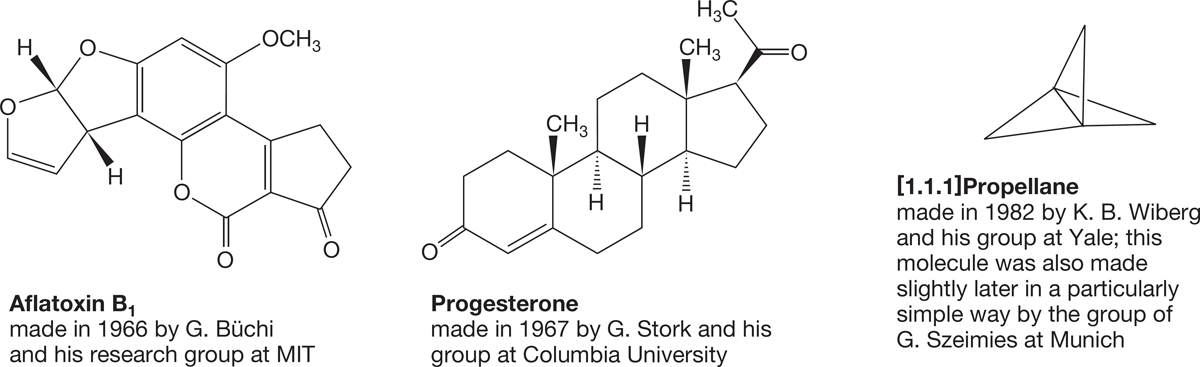
FIGURE 2.53 Some polycyclic molecules.
Summary
To name alkanes:
1. Find the longest chain and use the appropriate root word.
2. Number the chain to give the lowest possible numbers for the substituents.
3. Arrange the substituents in the prefix alphabetically, and include the carbon number for each to describe its location.
4. Use the alphabetical preference rule only if necessary.
Drawing isomers requires finding a system that allows you to consider all the possible perturbations. One approach is to start with the longest possible chain and then reduce the chain length one carbon at a time, considering the possible locations of the displaced methyl group with each reduction.
Cycloalkanes are bonded in the same way as noncyclic alkane molecules—by overlap of sp3 hybrid orbitals. Ring compounds have sides, which means that substituents can be on the same side (cis) or on opposite sides (trans). All manner of polycyclic molecules (two or more rings) exist.