6.1 Preview
In this chapter, we will see some old friends we met in Chapters 2 and 3, alkyl halides and alcohols, as well as some new molecules rather closely related to them: amines, ethers, thiols, and thioethers. This structural diversity is both a blessing and a curse. The various functional groups of organic chemistry lead to a multitude of possible chemical reactions and, ultimately, to biological complexity—including we humans.
The language of organic chemistry requires an understanding of the nomenclature of the organic molecules. We will learn how to name the substituted alkanes. This is not too difficult, as long as you avoid errors in counting, as aptly demonstrated by King Arthur in the opening quote.
We have already learned that alkyl halides and alcohols can be made through the addition reactions discussed in Chapter 3 (p. 135). Amines somewhat resemble alcohols, and understanding the chemistry of amines is a necessary prelude to working through Chapter 21, which discusses the chemistry of some of the many biologically important molecules containing nitrogen. A nitrogen atom provides one of the crucial linking agents in the formation of polyamino acids, called peptides or proteins (Fig. 6.1). In this chapter, we examine the qualities that make amines, alcohols, and thiols similar and prepare for Chapters 7 and 8, where we will take a long, detailed look at four prototypal new reactions in which alkyl halides and alcohols are especially important.
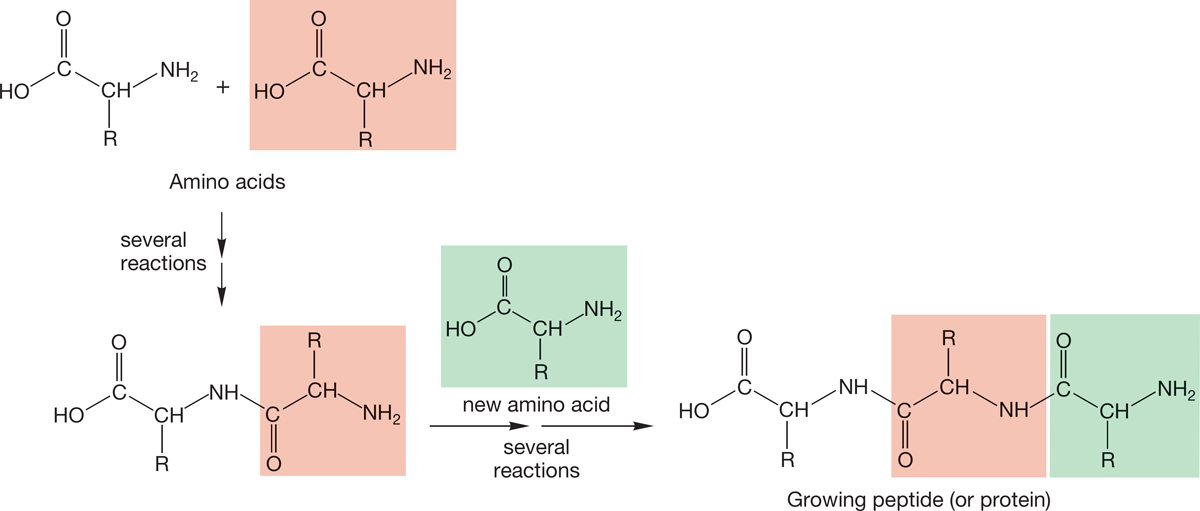
FIGURE 6.1 A nitrogen-to-carbon link is vital in the natural polymeric molecules known as proteins or peptides.
ESSENTIAL SKILLS AND DETAILS
1. Much of chemistry, including organic chemistry, involves acids and bases. In this chapter, we review what we know already about this subject and focus on a measure of acidity, pKa. It is important to have a reasonable idea of the pKa values of the general classes of compounds. A Problem Solving box on determination of relative acidity appears later in this chapter, and a table of pKa values is on the inside of the back cover of this book.
2. A low pKa means that the molecule in question is a strong acid, a good proton donor. Conversely, a high pKa means that the compound is a poor acid.
3. You also learn about solvation in this chapter. Being able to choose an appropriate solvent for a reaction and to understand how solvents operate is an important skill both in the practical world and in this course.
4. You will study organometallic reagents, including Grignard reagents, in this chapter. The synthetic sequence “alkene → halide → Grignard reagent” is one you will use many times in solving synthetic problems.