6.9 Special Topic: Crown Ethers
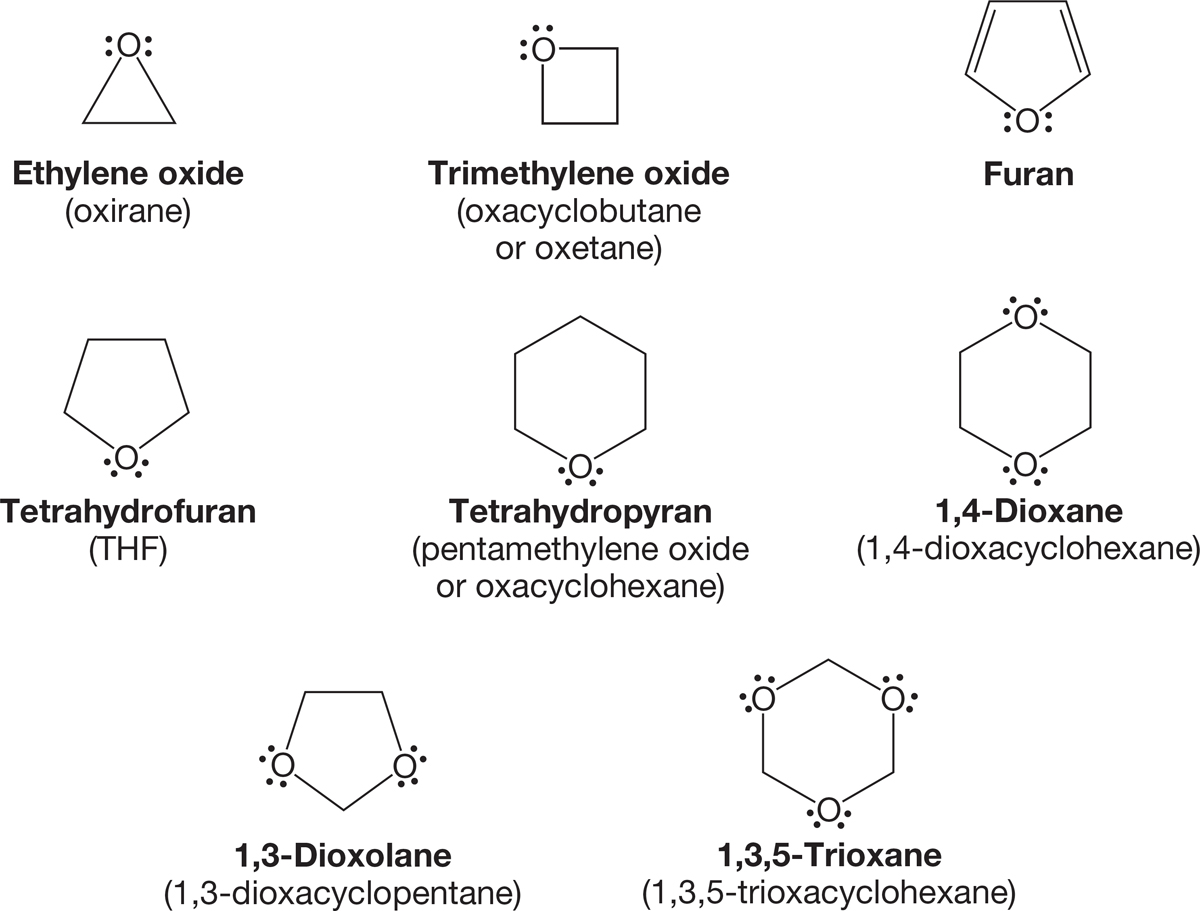
Cyclic ethers are common solvents and, as we will see in Section 7.9b, can often be prepared rather easily (Williamson ether synthesis, p. 318). It is possible to make cyclic polyethers, compounds in which there is more than one ether linkage. Figure 6.14 (p. 237) gives some examples of cyclic ethers and cyclic polyethers.
We have mentioned more than once the remarkable solvating powers of ethers. Ethers are Lewis bases and can donate electrons to Lewis acids, thus stabilizing them. One example of this stabilization is the requirement for an ether, usually diethyl ether or THF, in the formation of a Grignard reagent. Ethers are also slightly polar compounds and therefore are able to stabilize other polar molecules through noncovalent dipole–dipole interactions.
This stabilization was carried to extremes in work that ultimately led to the 1987 Nobel Prize in Chemistry for Charles J. Pedersen (1904–1989) of DuPont, Donald J. Cram (1919–2001) of the University of California, Los Angeles, and Jean-Marie Lehn (b. 1939) of Université Louis-Pasteur in Strasbourg for opening the field of “host–guest” chemistry. Pedersen discovered that certain cyclic polyethers (the hosts) had a remarkable affinity for metal cations (the guests). Molecules were constructed whose molecular shapes created different-sized cavities into which different metal ions fit well. Because of their vaguely crown-shaped structures, these molecules came to be called crown ethers. Figure 6.65 shows two of them.
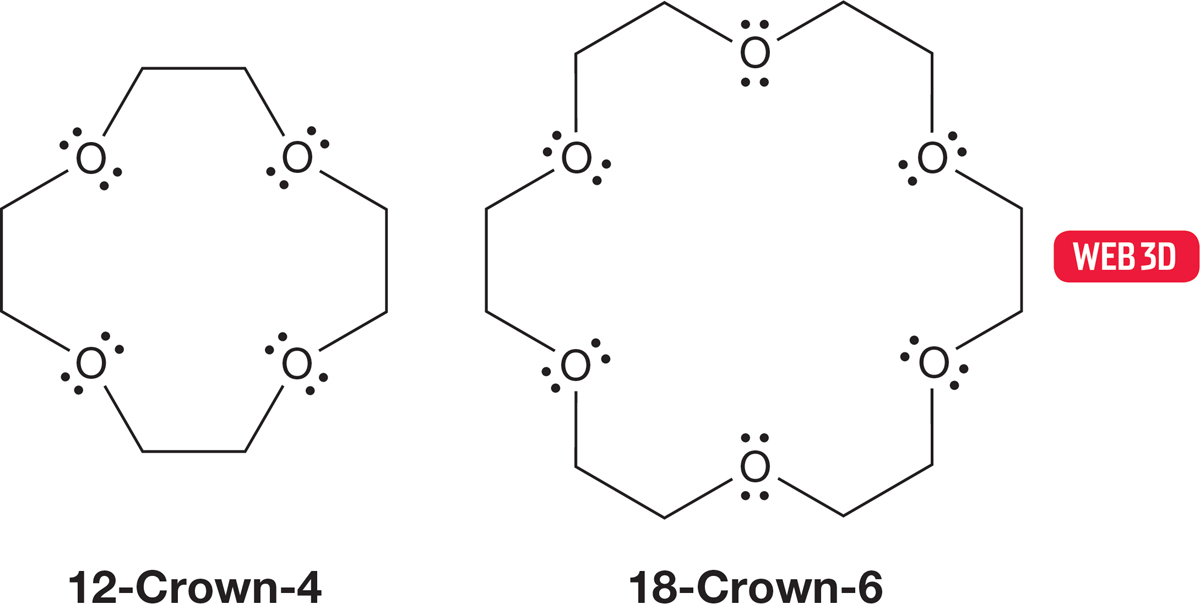
FIGURE 6.65 Two crown ethers. The first number in the name shows the number of atoms in the ring, and the final number shows the number of heteroatoms in the ring. In these molecules the heteroatoms are all oxygen, but others are possible.
The molecule 12-crown-4 is the proper size to capture lithium ions, and 18-crown-6 has a remarkable affinity for potassium ions. These crown ethers can stabilize positive ions of different sizes, depending on the size of the cavity (Fig. 6.66).
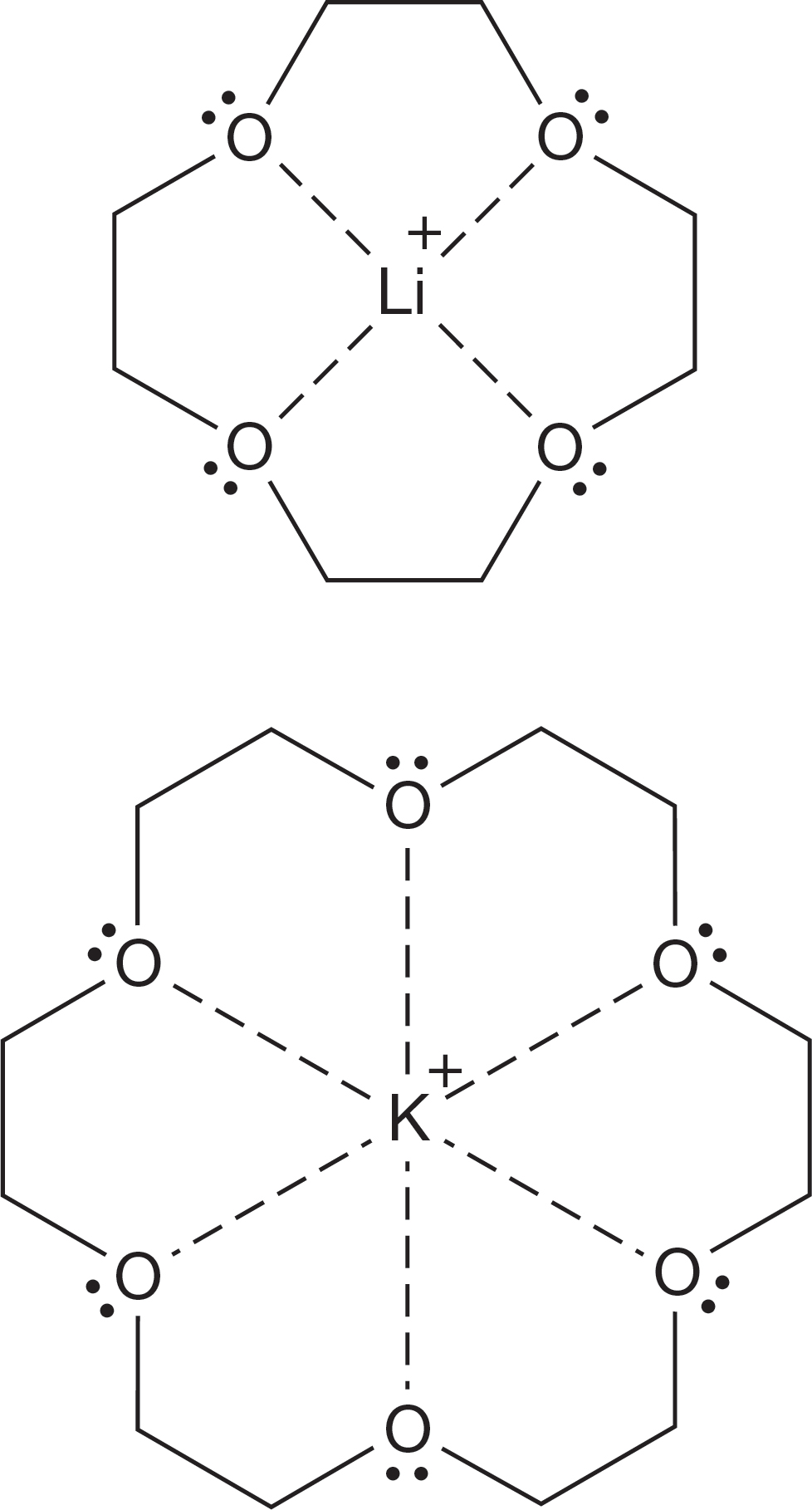
FIGURE 6.66 Two crown ethers stabilizing metal cations.
Three-dimensional versions of these essentially two-dimensional molecules have now also been made using both oxygen and other heteroatoms, such as sulfur and nitrogen, as the Lewis bases. These host molecules are called cryptands and can incorporate positive ions into the roughly spherical cavity within the cage (Fig. 6.67).
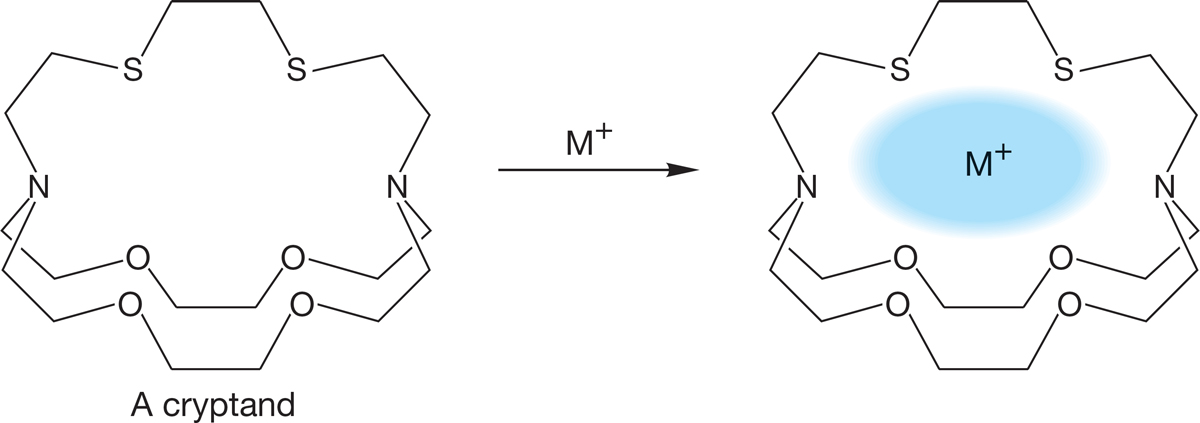
FIGURE 6.67 Three-dimensional cryptands can incorporate metal ions into a central cavity.
Research in the area of crown ethers has produced significant results. For example, potassium permanganate (KMnO4) is an effective oxidizing agent, but its great insolubility in organic reagents limits its use. The deep-purple KMnO4 is completely insoluble in benzene. It simply forms a solid suspension when added to benzene, which remains a colorless liquid. However, the addition of a little 18-crown-6 results in the dissolution of the permanganate, and the benzene takes on the deep purple color of the permanganate ion. The potassium ion has been effectively solvated by the crown ether and now dissolves in the organic solvent. The permanganate counterion, though not solvated by the crown compound, accompanies the positive ion into solution, as it must to preserve electrical neutrality. The purple benzene solution can now be used as an effective oxidizing agent.
Schemes have been envisioned in which specially designed crown compounds are used to scavenge poisonous heavy metal ions from water supplies. There have been less humanitarian, though admittedly clever, schemes for extracting the minute amounts of gold ions present in seawater by passing vast amounts of water through a column of sand impregnated with a gold-selective crown compound.