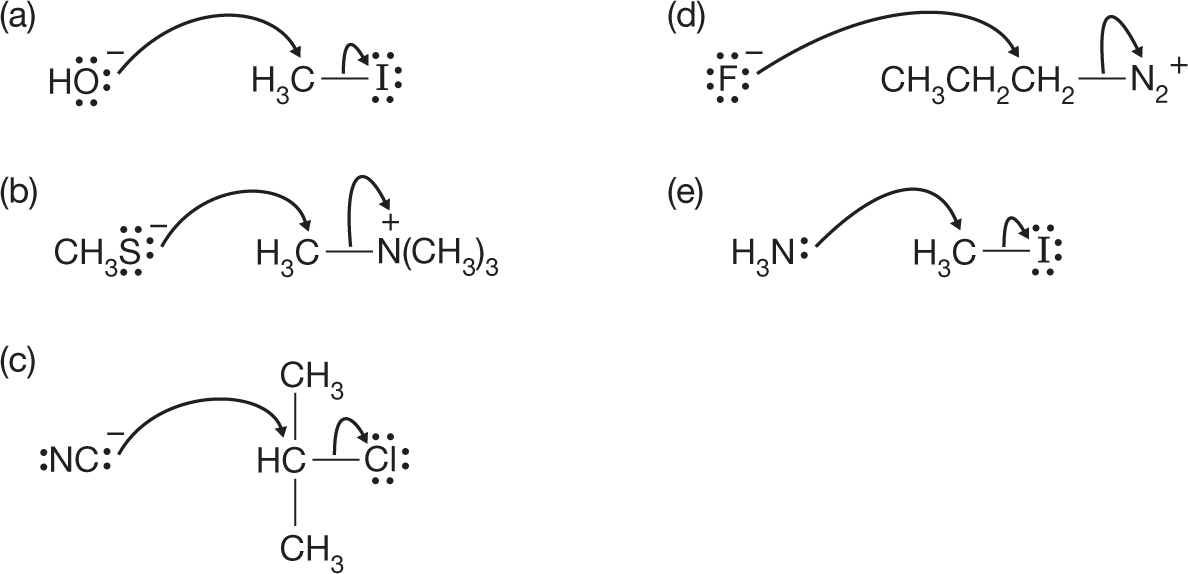
7.3 Reactions of Alkyl Halides: The Substitution Reaction
A great many varieties of substitution reactions are known, but all involve a competition between a pair of Lewis bases for a Lewis acid. Figure 7.9 shows five examples with the substituting group, the nucleophile (from the Greek, “nucleus loving”), and the displaced group, the leaving group, identified. Both the nucleophile (usually Nu) and leaving group (usually LG) are Lewis bases.
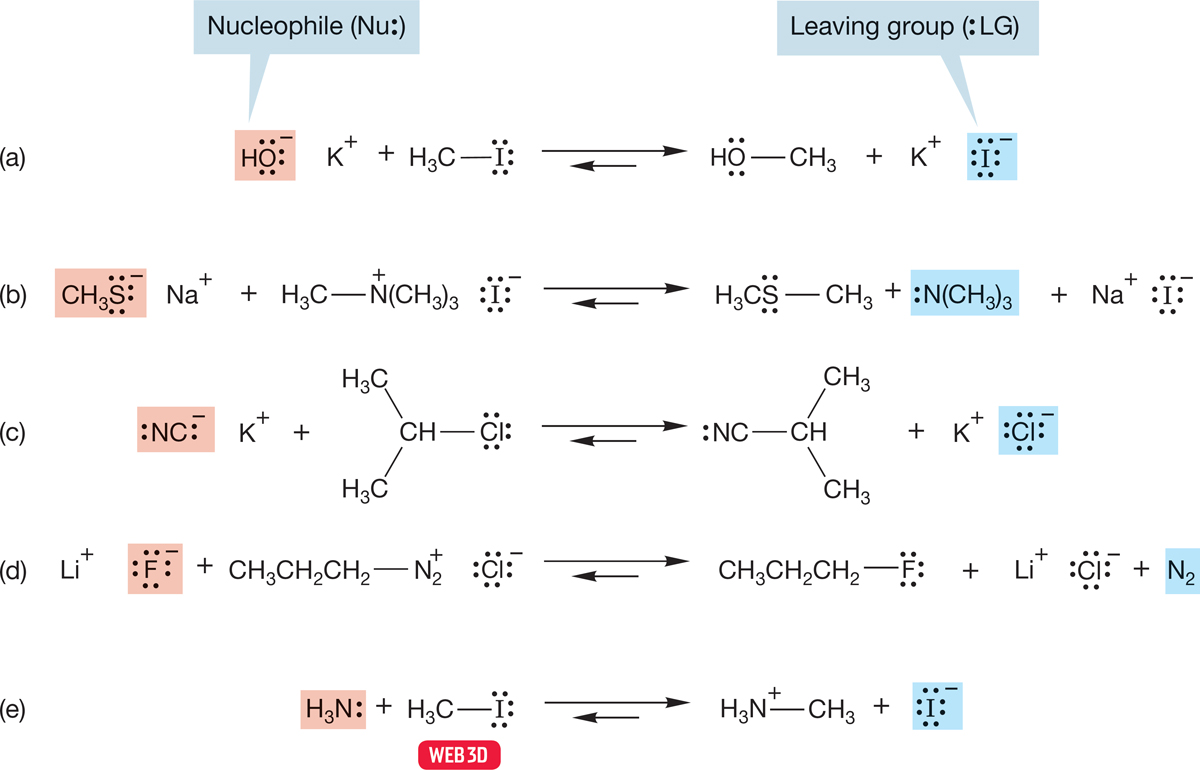
FIGURE 7.9 Five examples of the substitution reaction. The nucleophiles for the reactions reading top to bottom are (a) hydroxide (HO−), (b) methyl mercaptide (CH3S−), (c) cyanide (NC−), (d) fluoride (F−), and (e) ammonia (:NH3). The leaving groups are iodide (I−), trimethylamine [N(CH3)3], chloride (Cl−), nitrogen (N2), and iodide (I−) again.
Notice that the reactions in Figure 7.9 are all equilibrium reactions. These competitive reactions are all, in principle at least, reversible. A given reaction may not be reversible in a practical sense, but that simply means that one of the competitors has been overwhelmingly successful. There are many possible reasons why this might be so. One of the equilibrating molecules might be much more stable than the other. If one of the nucleophiles were much more powerful than the other, the reaction would be extremely exothermic in one direction or the other. Reactions a–c in Figure 7.9 are examples of this. We are used to thinking of reactions running from left to right, but this prejudice is arbitrary and even misleading. Reactions run both ways.
There are other reasons that one of these reactions is irreversible. Perhaps the equilibrium is disturbed by one of the products being a solid that crystallizes out of the solution or a gas that bubbles away. If so, the reaction can be driven all the way to one side, even against an unfavorable equilibrium constant. Under such circumstances, an endothermic reaction can go to completion. In reaction d of Figure 7.9, both propyl fluoride (boiling point 2.5°C) and nitrogen are gases, and their removal from the solution drives the equilibrium to the right.
Another point to notice is that there is no obligatory charge type in these reactions. The displacing nucleophile is not always negatively charged (reaction e), and the group displaced—the leaving group—does not always appear as an anion (reactions b and d).
If we examine a large number of substitution reactions, two categories emerge. The boundaries between them are not absolutely fixed, as we will see, but there are two fairly clear-cut limiting classes of reaction. One is a kinetically second-order process, common for primary and secondary substrates (Section 7.5). The other is a kinetically first-order reaction, common for tertiary substrates (Section 7.7). We take up the first of these two reactions in Section 7.5, moving on to the other in Section 7.7. In any discussion of reaction mechanisms, of how chemical reactions occur, questions of energy are critical. Therefore, we take the next several pages to review energy, equilibrium (thermodynamics), and reaction rates (kinetics).