2.18 Additional Problems
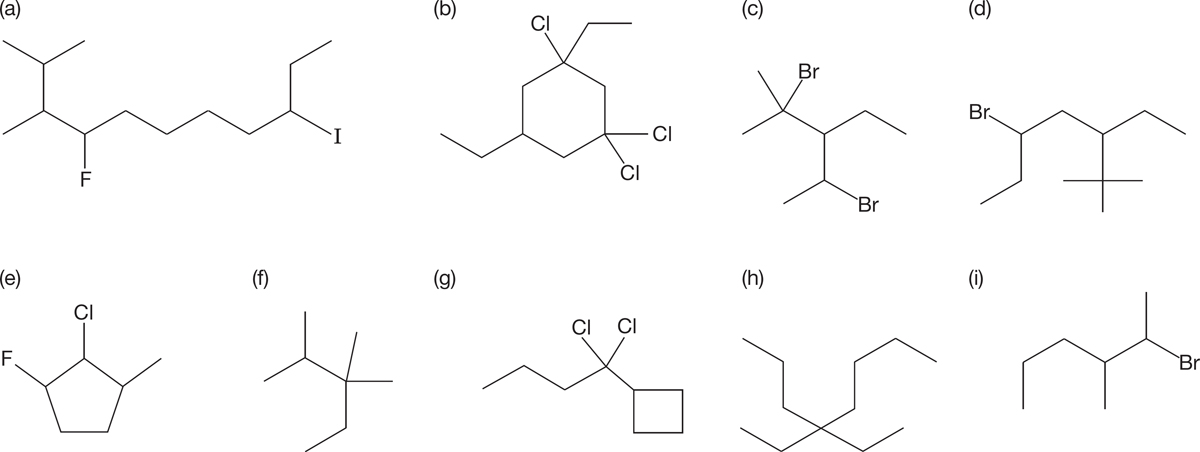
PROBLEM 2.41 Provide the IUPAC name for each of the following compounds:
PROBLEM 2.42 Write Lewis structures for Et2O, CH3CO2H, CH3CHOHCH3, CH2F2, CH3CH2SCH3, CH3(CH2)3OH, MeCN, and (CH3)2CO. Show nonbonding electrons as dots and electrons in bonds as lines.
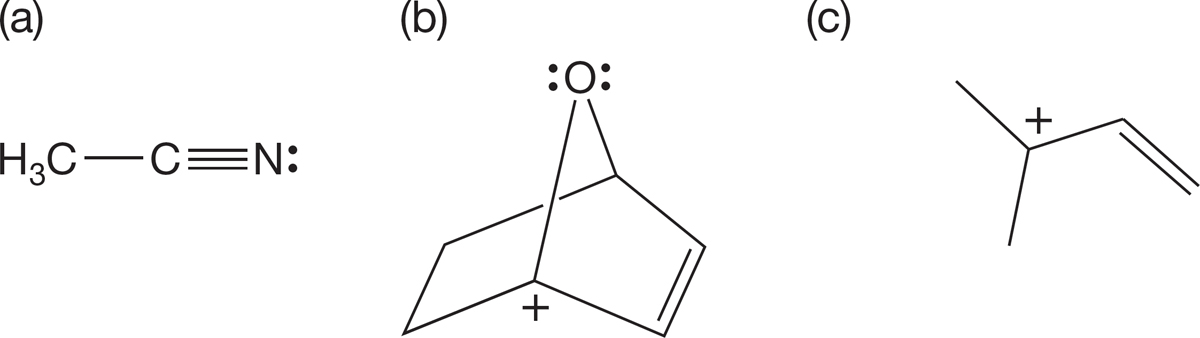
PROBLEM 2.43 Assign hybridization to the C, N, and O atoms in the following structures:
PROBLEM 2.44 Redraw the following line structures so that each atom (including hydrogens), each bond, and any lone pairs are clearly shown.
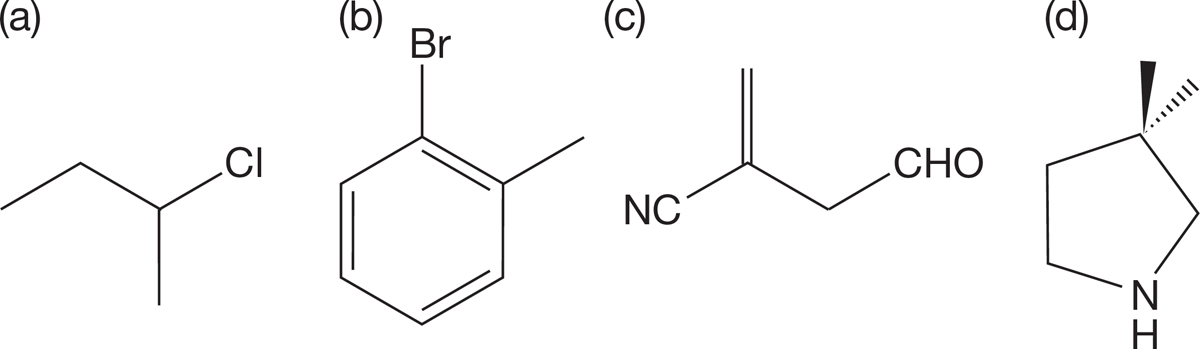
PROBLEM 2.45 Circle and name the functional groups in each of the molecules shown in Problem 2.44.
PROBLEM 2.46 Draw all the isomers of C5H11Cl. Give proper systematic names to all of them. Hint: There are eight isomers.
PROBLEM 2.47 Use your model set to look down the C(3)―C(4) bond of hexane. Draw the Newman projections for the three staggered and the three eclipsed conformations.
PROBLEM 2.48 Use your model set to look down the C(2)―C(3) bond of 2-bromo-3-methylbutane. Draw the Newman projection for all possible staggered conformations. Circle the one you think is the most stable and explain why you’ve chosen that one. Determine the number of gauche interactions in each projection.
PROBLEM 2.49 Draw the Newman projections for the different eclipsed and staggered conformations of 2,3-dichlorobutane. Look down the bond joining the two chlorine-bearing carbons. Label each projection as either eclipsed or staggered. In each staggered projection, determine the number and type of gauche interactions.
PROBLEM 2.50 Draw Newman projections constructed by looking down the C(1)―C(2) bond of 2-methylpentane. Repeat this process looking down the C(2)―C(3) bond. In each case, indicate which conformations will be the most stable.
PROBLEM 2.51 Although a chlorine is about the same size as a methyl group, the conformation of 1,2-dichloroethane in which the two chlorines are eclipsed is higher in energy than the conformation of butane in which the two methyl groups are eclipsed. Explain. Hint: Size isn’t everything!
PROBLEM 2.52 What is the approximate hybridization of the indicated carbon in the following compounds?
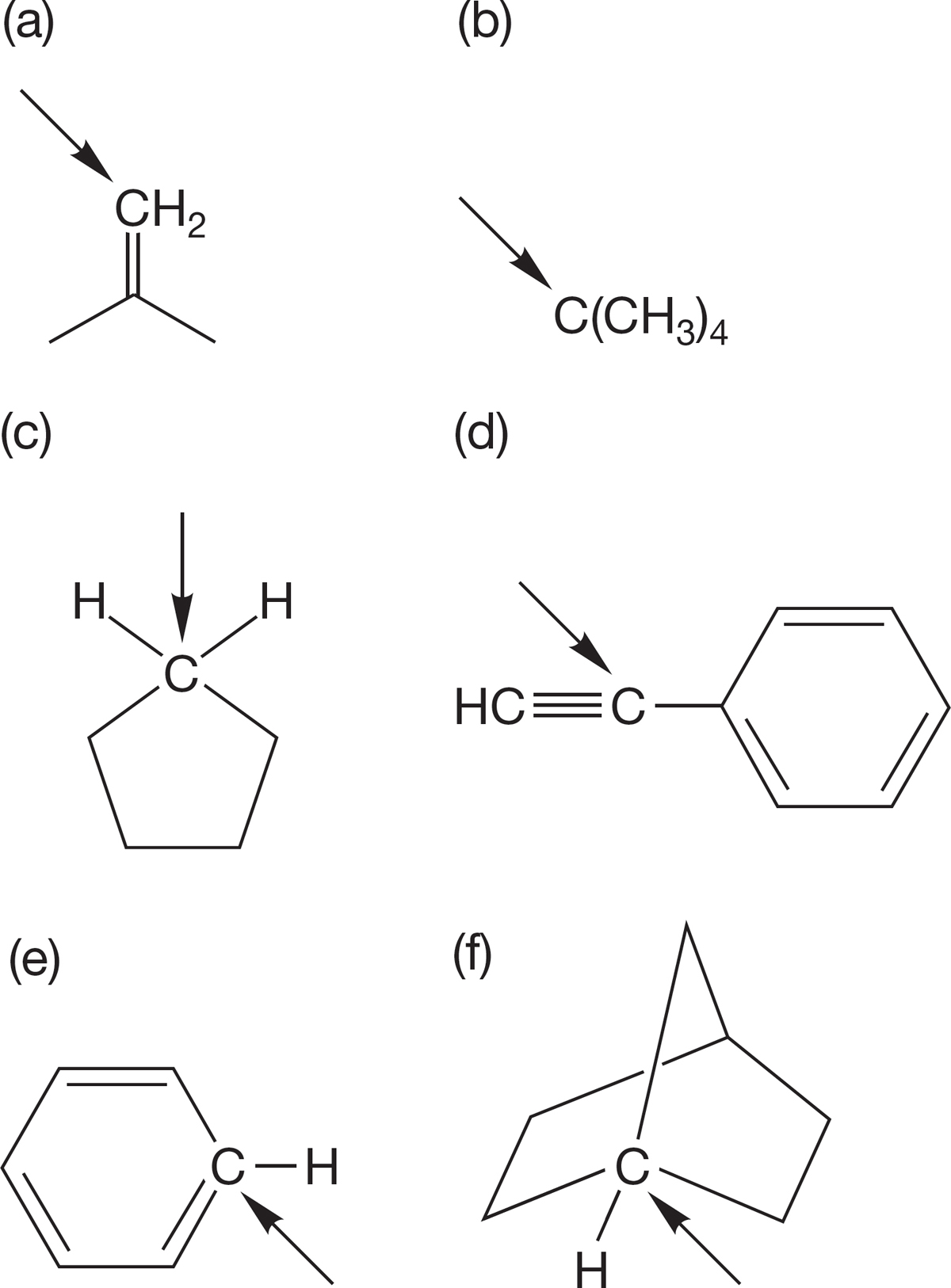
PROBLEM 2.53 Indicate the hybridization for each carbon, nitrogen, and oxygen in the molecules shown below. Put a circle around the sp3-hybridized atoms, a triangle around sp2- hybridized atoms, and a box around the sp-hybridized atoms.
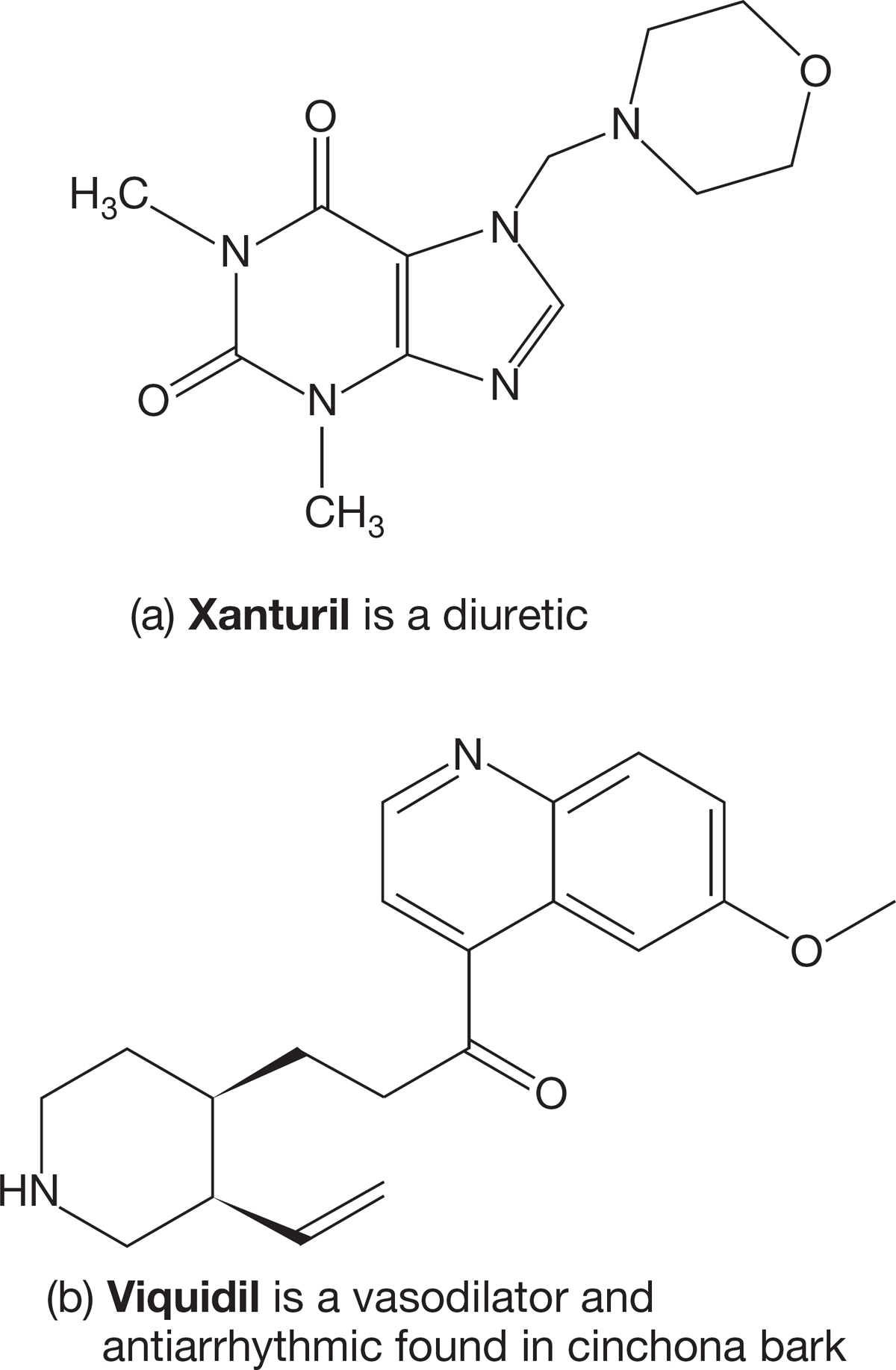
PROBLEM 2.54 Use the boiling point data in Table 2.4 to estimate the boiling point of pentadecane, C15H32.
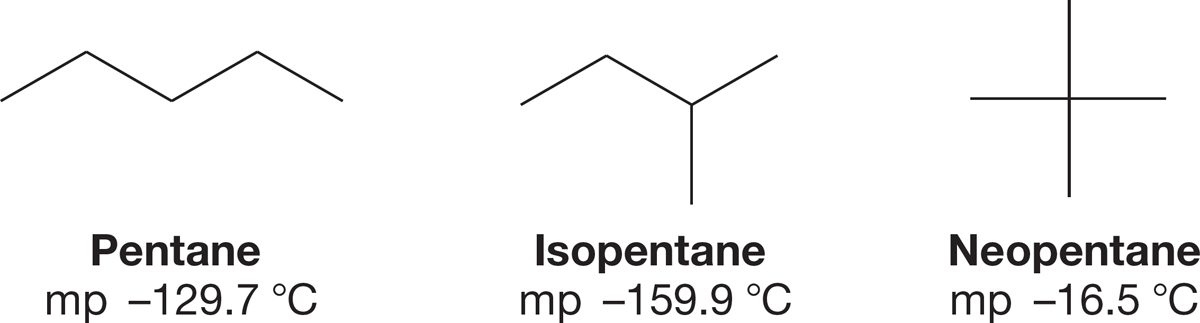
PROBLEM 2.55 Rationalize the differences in the melting points for the isomeric pentanes shown below.
PROBLEM 2.56 Imagine replacing two hydrogens of a molecule with some group X. For example, methane (CH4) would become CH2 X2. (a) What compounds would be produced by replacing two hydrogens of ethane? (b) What compounds would be produced by replacing two hydrogens of propane? (c) What compounds would be produced by replacing two hydrogens of butane?
PROBLEM 2.57 Draw a molecule that has the formula of C8H14 and has only two four-membered rings. How many different carbons are there in the molecule you have drawn?
PROBLEM 2.58 How many signals would a 13C NMR spectrometer “see” for (a) pentane, (b) isopentane, and (c) neopentane (see Figure 2.36)?
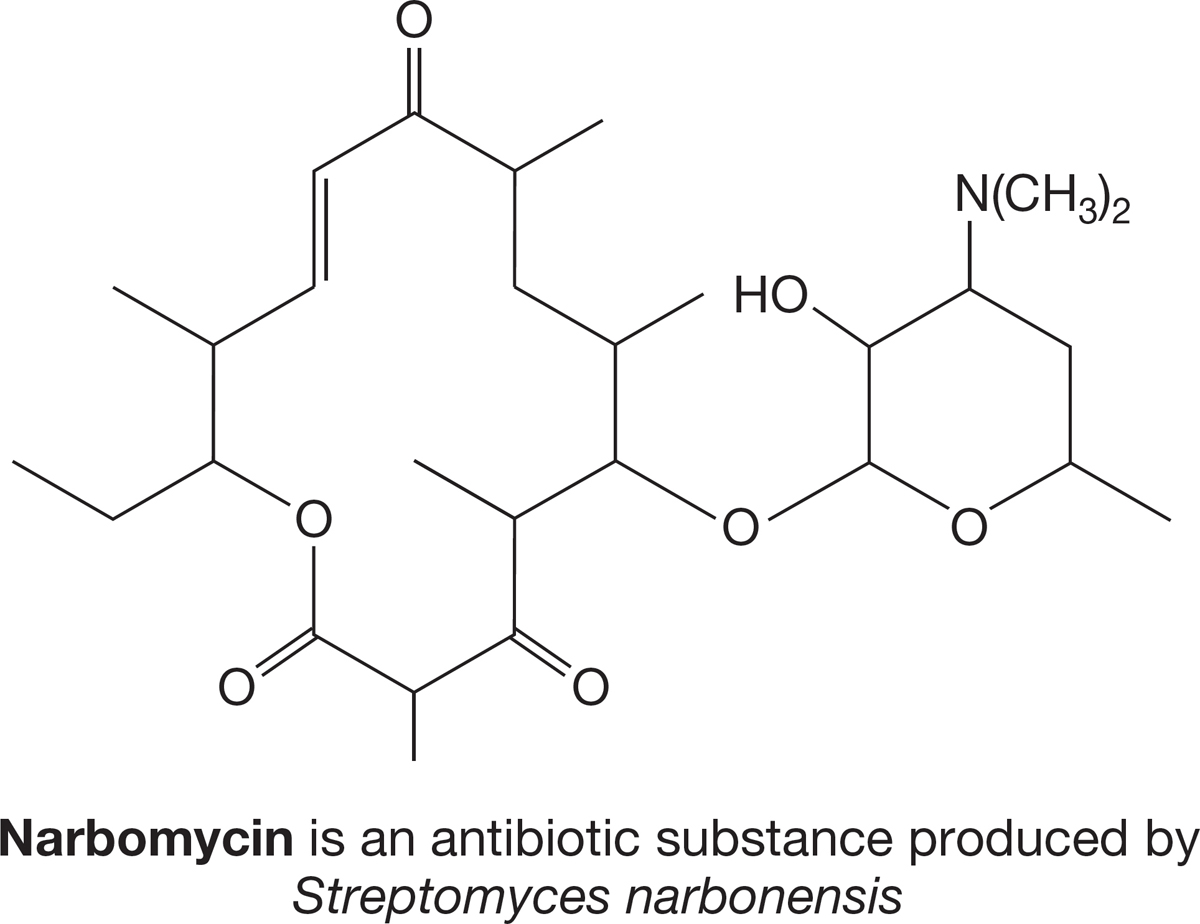
PROBLEM 2.59 Circle and name eight functional groups in Narbomycin.
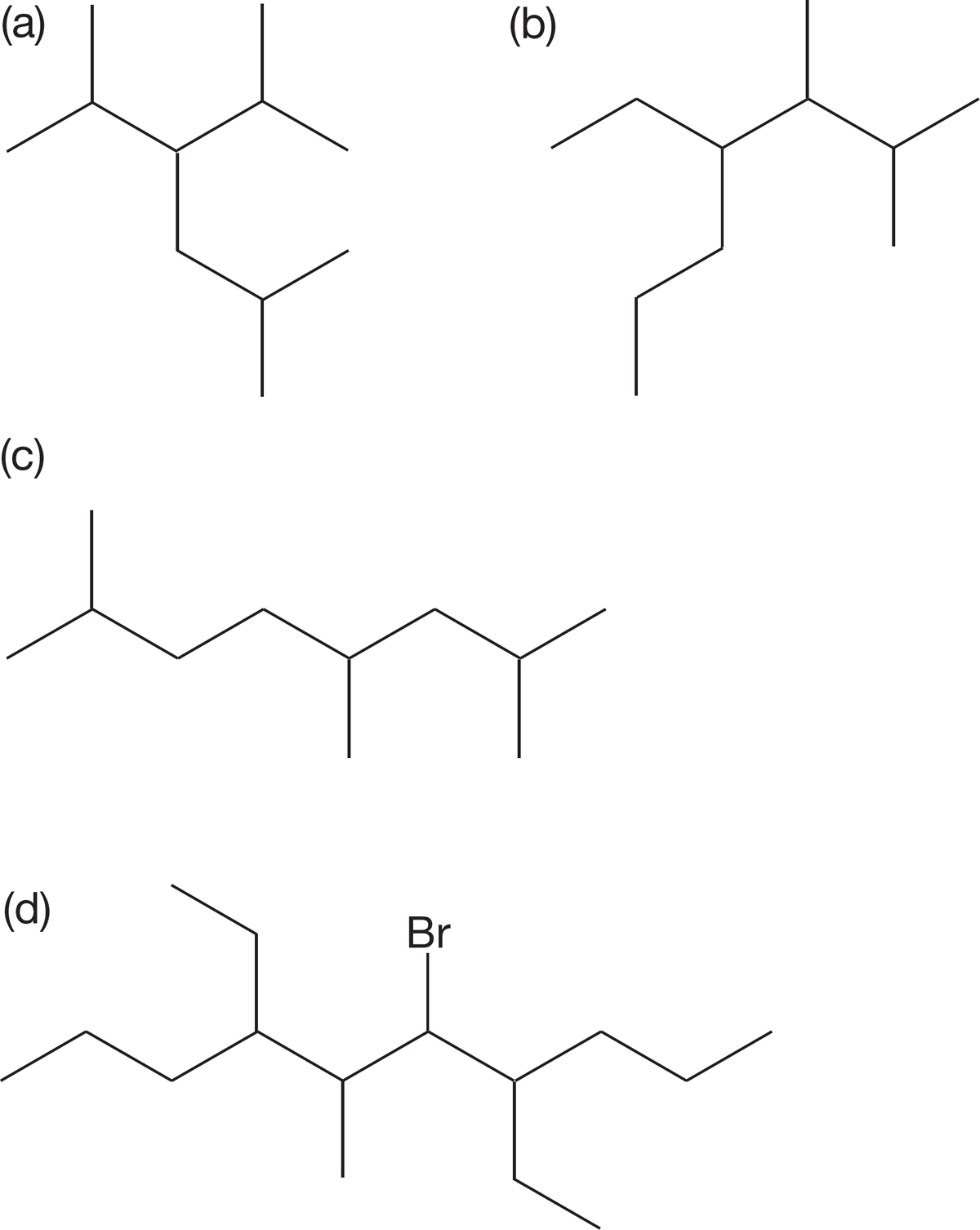
PROBLEM 2.60 Write systematic names for the following compounds:
PROBLEM 2.61 Draw structures for the following compounds:
(a) 4-ethyl-4-fluoro-2-methylheptane
(b) 2,7-dibromo-1-chloro-4-propyloctane
(c) 3,7-diethyl-2,2-dimethyl-4-propylnonane
(d) 1,1,3-trimethylcyclopentane
PROBLEM 2.62 Although you should be able to draw structures for the following compounds, each is named incorrectly below. Give the correct systematic name for each of the following compounds:
(a) 2-methyl-2-ethylpropane
(b) 2,6-diethylcyclohexane
(c) 1-bromo-3-isopropylpentane
(d) 5-fluoro-8-methyl-3-tert-butylnonane
PROBLEM 2.63 One of the octane isomers of Problem 2.28, 3-ethyl-2-methylpentane, could have been reasonably named otherwise. Draw this compound and provide the alternative nonsystematic name.
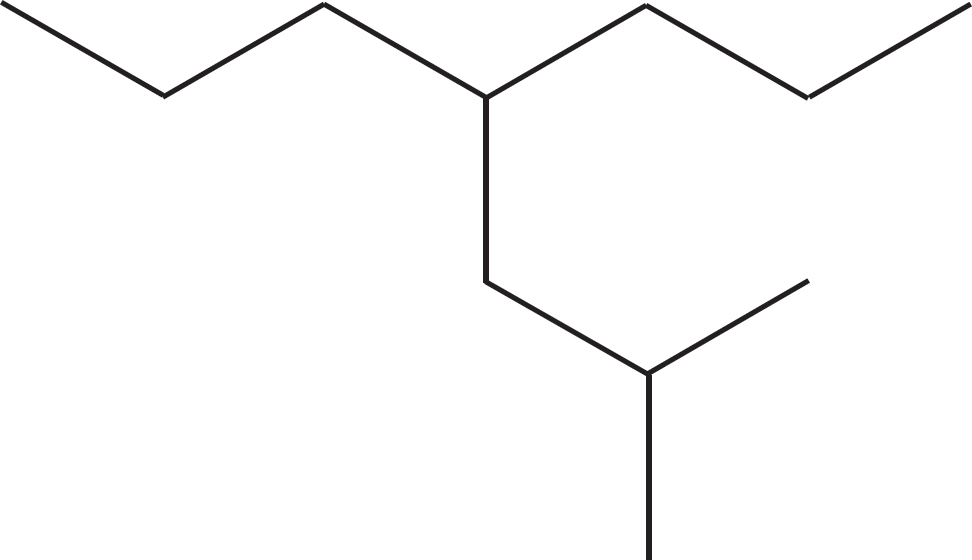
PROBLEM 2.64 Name the following compound:
PROBLEM 2.65 Draw 1-bromobutane and 2-bromobutane. How many primary, secondary, and tertiary carbons are there in each molecule you have drawn?
PROBLEM 2.66 We often refer to hydrogens on a primary carbon as primary hydrogens. In the same way, a secondary hydrogen is on a secondary carbon, and a tertiary hydrogen is on a tertiary carbon. How many primary, secondary, and tertiary hydrogens are present in 3-chlorohexane?
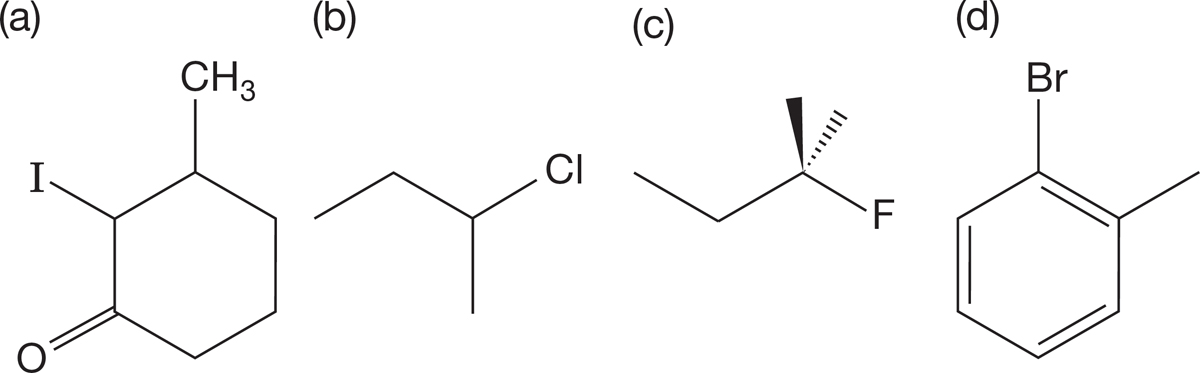
PROBLEM 2.67 A primary halide is an alkyl halide that has the halogen attached to a primary carbon. A secondary halide has the halogen attached to a secondary carbon, and a tertiary halide has the halogen attached to a tertiary carbon. Identify the type (primary, secondary, or tertiary) of halide in each of the following structures:
PROBLEM 2.68 Show the curved arrow formalism (electron pushing or arrow pushing) for the protonation of water in sulfuric acid (HOSO2OH = H2SO4).
PROBLEM 2.69 Show the curved arrow formalism (electron pushing or arrow pushing) for the protonation of ammonia (NH3) in hydrochloric acid.
PROBLEM 2.70 Show the curved arrow formalism for the protonation of CH3CH2OH in HCl.
PROBLEM 2.71 Show the curved arrow formalism for the protonation of CH3CH2OCH2CH3 in H2SO4. Show the electron pushing for the reverse of this reaction.
Use Organic Reaction Animations (ORA) to answer the following questions:
PROBLEM 2.72 Choose the reaction titled “SN2 with cyanide” and click on the Play button. As you watch the reaction occur, notice that the methyl group on the left rotates during the process. Explain why there is rotation.
PROBLEM 2.73 Choose the reaction titled “Alkene hydrohalogenation” and click on the Play button. Notice that there are two steps to this reaction. Identify the electrophile and the nucleophile in the first step. Identify the electrophile and the nucleophile in the second step.
PROBLEM 2.74 Choose the reaction titled “Bimolecular nucleophilic substitution: SN2” and click on the Play button. Notice that the SN2 is a one-step reaction. What is the nucleophile in this reaction? You can click on the highest occupied molecular orbital (HOMO) button to observe the electron density of the nucleophile. What is the electrophile? The electrophile is shown in the lowest unoccupied molecular orbital (LUMO) movie.
If your instructor assigns problems in  , log in at smartwork.wwnorton.com.
, log in at smartwork.wwnorton.com.