2.4 The Methyl Cation (+CH3), Anion (-:CH3), and Radical (·CH3)
The molecules +CH3, -:CH3, and ·CH3 are members of a class of compounds called reactive intermediates. This name implies that these molecules are too unstable to be isolable under normal conditions and must usually be studied by indirect means. Often, this means looking at what they did during their brief lifetimes or sometimes isolating them at very low temperature. These three species are especially important because they are prototypes—the simplest examples of whole classes of molecules to be encountered later when our attention turns to the study of chemical reactions.
We call +CH3 a methyl cation. To make this molecule, you could imagine removing a hydride (-:H) from methane to produce+CH3. It is the simplest example of a carbocation, a molecule containing a positively charged carbon3 (Fig. 2.14a).
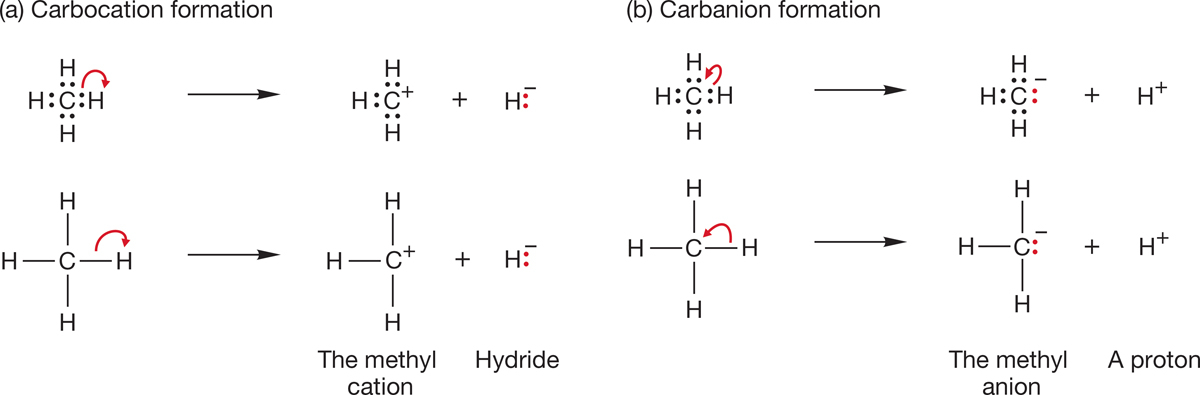
FIGURE 2.14 The formation of (a) the methyl cation (+CH3) and (b) the methyl anion (-:CH3) by two different heterolytic cleavages of a carbon–hydrogen bond in CH4. The top example in each case shows the bonding electrons and the path they take. The bottom examples will be the typical way of representing heterolytic bond cleavage.
Now imagine forming the methyl anion (-:CH3) by removing a proton (H+) from methane (Fig. 2.14b), leaving behind a pair of nonbonding or lone-pair electrons in the negatively charged methyl anion. The resulting -:CH3 is a simple example of a carbon-based anion, or carbanion.
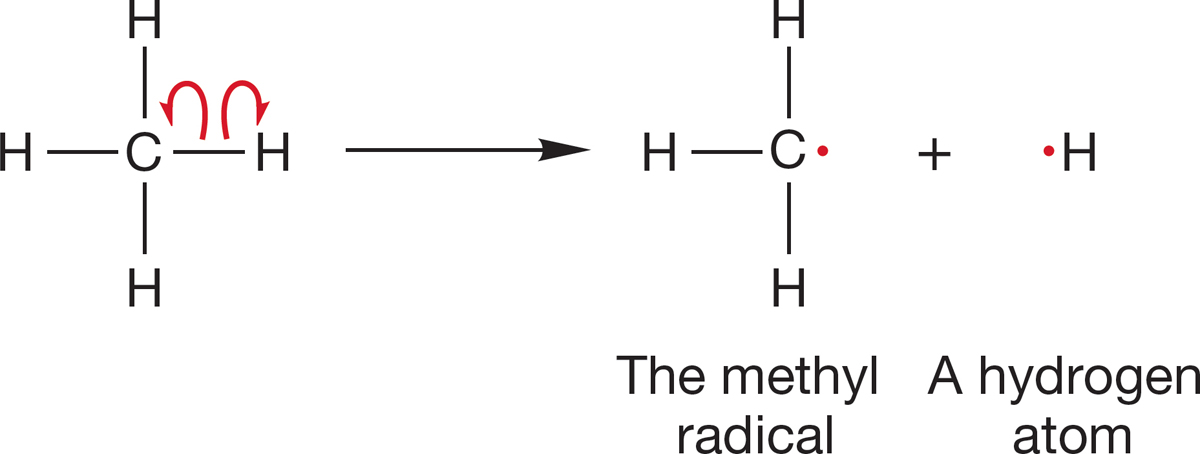
FIGURE 2.15 The homolytic cleavage of a carbon–hydrogen bond in methane to give a hydrogen atom and the methyl radical.
Both of these ways of producing +CH3 and -:CH3 involve the concept of breaking a carbon–hydrogen bond in unsymmetrical fashion, a process known as heterolytic bond cleavage (p. 39 and Fig. 2.14). Remember the curved arrow formalism—the red arrows of Figure 2.14 move the pair of electrons in the carbon–hydrogen bond to the hydrogen or to the carbon.
Recall from p. 39 that there is another way of breaking a two-electron bond, and that is to allow one electron to go with each atom associated with the breaking bond (Fig. 2.15). This homolytic bond cleavage in methane gives a hydrogen atom (H·) and leaves behind the neutral methyl radical (·CH3). A radical is an atom or molecule that has one (or more) unpaired electrons. Chapter 12 is devoted to the topic of the chemistry of radicals. Note the single-barbed (“fishhook”) curved arrow convention used to represent movement of one electron.
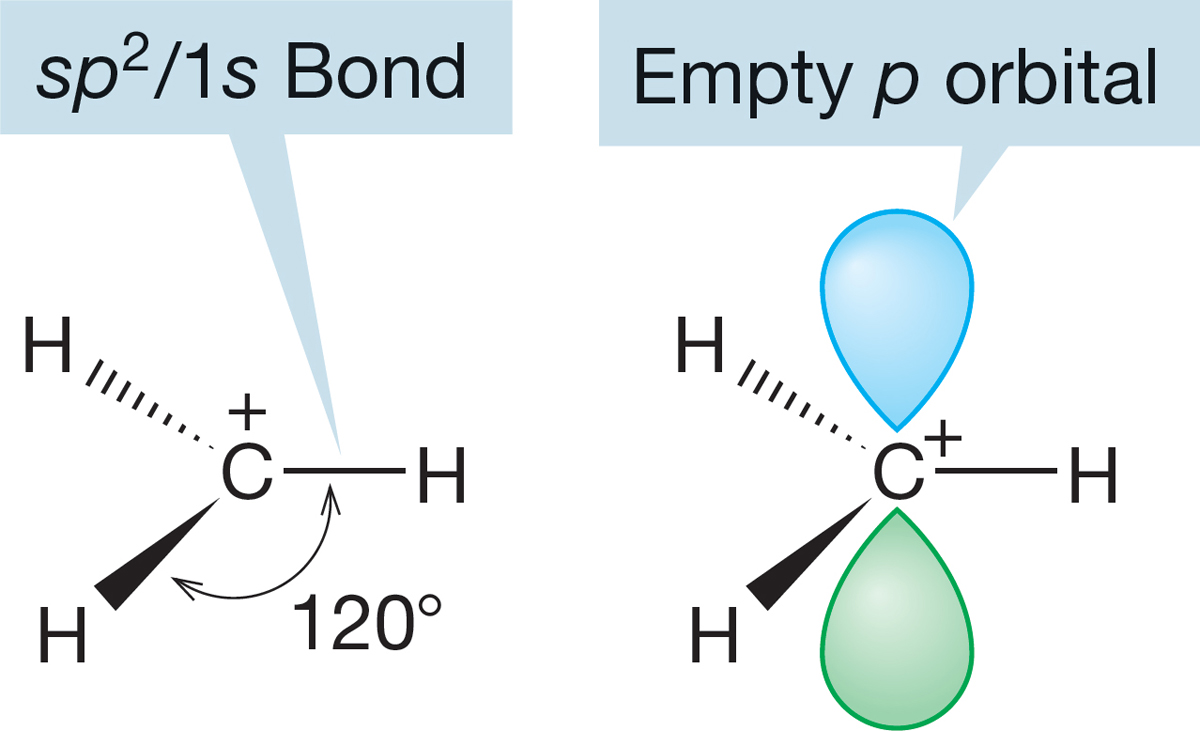
FIGURE 2.16 The sp2-hybridized methyl cation, +CH3. The three bonds shown are the result of overlap between carbon’s sp2 hybrid orbitals and the 1s atomic orbital of each hydrogen. The four atoms all lie in the same plane, which is perpendicular to the plane of the page.
The methyl cation, anion, and radical have all been observed, although each is extremely reactive and thus short-lived. They exist, though, and we can make some predictions of structure for at least two of them. In the methyl cation (CH3), carbon is attached to three hydrogens, suggesting the need for three hybrid atomic orbitals (recall BH3, p. 59), and therefore sp2 hybridization (Fig. 2.16).
Unlike the methyl cation, the carbon in the methyl anion not only is attached to three hydrogens but also has a pair of nonbonding electrons. The cation has an empty pure p orbital (zero s character), and therefore the species is as flat as a pancake (H―C―H angle = 120°). The methyl anion has two more electrons than the cation, and we have to consider them in arriving at a prediction of the anion’s shape. Recall from Figure 1.7 that s orbitals have density at the nucleus. Because the nucleus is positively charged and electrons are negatively charged, it is reasonable to assume that an electron is more stable (lower in energy) in an orbital with a lot of s character. A pyramidal structure seems appropriate for the methyl anion, although it cannot be a perfect tetrahedron because this is a CH3 X molecule (where X is a lone pair of electrons). We can’t predict exactly how pyramidal the species will be, and an anion’s shape is difficult to measure in any case, but recent calculations predict the structure shown in Figure 2.17.
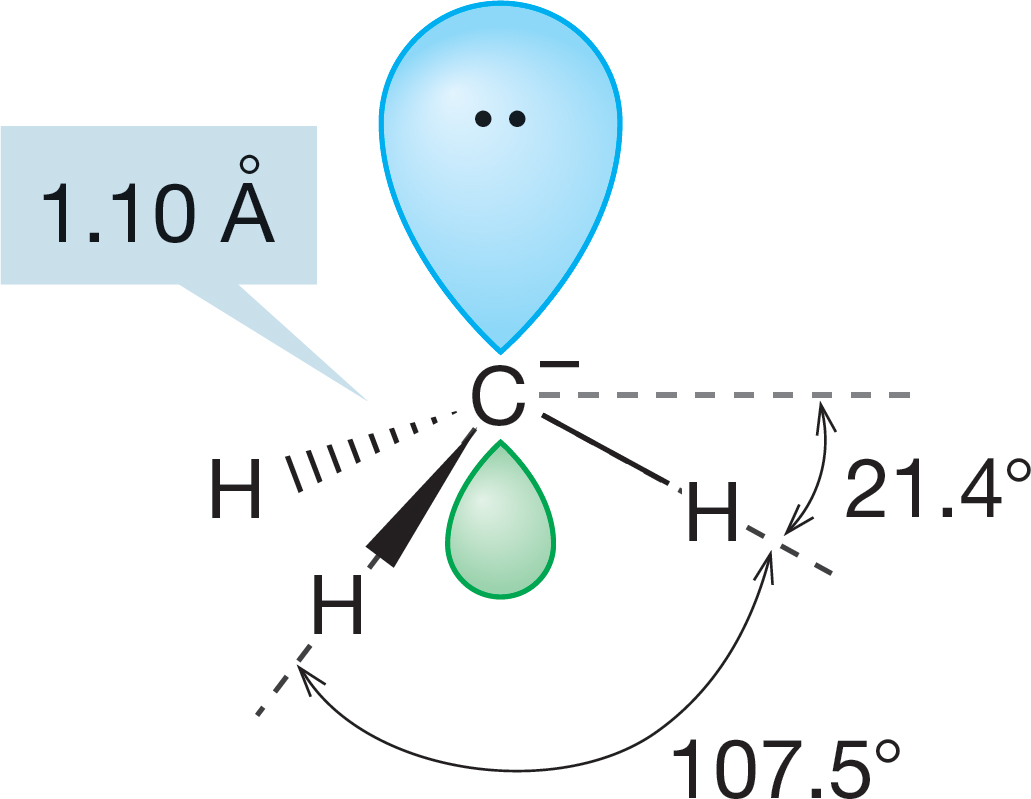
FIGURE 2.17 The structure of the methyl anion, -:CH3. The hybridization of the carbon in this carbanion is approximately sp3. The molecular shape is pyramidal. Each hydrogen is 21.4° below the horizontal plane.
It is harder to predict the structure of the neutral methyl radical (·CH3), in which there is only a single nonbonding electron. At present, it is not possible to choose between a planar species and a rapidly inverting and very shallow pyramid, although it is clear that the methyl radical is close to planar (Fig. 2.18). Do not be disturbed by this—chemists still do not know many seemingly simple things (such as the shape and hybridization of the methyl radical). There is still lots to do!
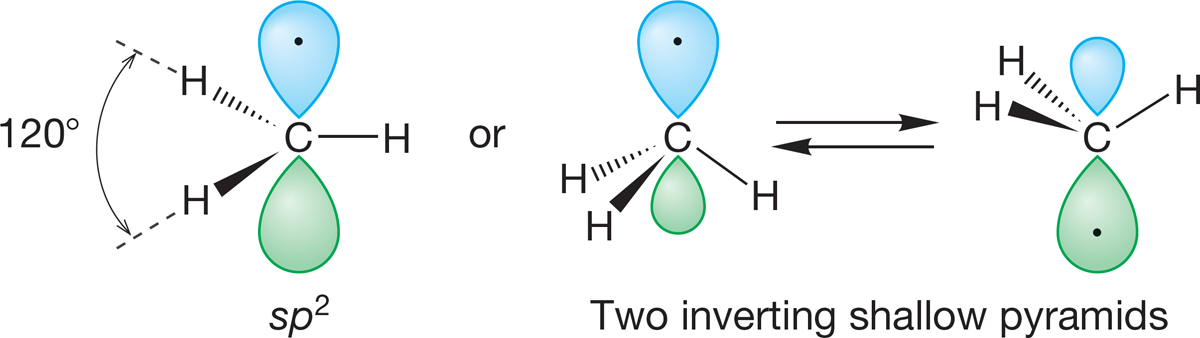
FIGURE 2.18 The methyl radical (·CH3) is either planar or a rapidly inverting shallow pyramid. The carbon is close to sp2 hybridized.
PROBLEM 2.11 Draw a structure for the methyl radical at the halfway point for the inversion shown in Figure 2.18. What is the hybridization of the carbon atom in the structure you drew?
Summary
Methane can be substituted in many ways through replacement of one or more hydrogens with another atom or groups of atoms. In principle, removal of a hydrogen from methane can lead to the methyl anion (-:CH3), the methyl radical (·CH3), or the methyl cation (+CH3), depending on the nature of the hydrogen removed (+H, ·H, or -:H). In this section, we have discussed only the shapes of these intermediates—reactions are coming later. It will be important to remember that carbocations are flat and sp2 hybridized and that simple carbanions are pyramidal and approximately sp3 hybridized.
3The name for carbocations was the focus of a long and too intense argument in the chemistry community. A carbocation is sometimes called a carbonium ion by traditionalists or a carbenium ion by others. The compromise carbocation is both aptly descriptive and avoids the emotional reactions of the staunch defenders of the other terms.