2.13 Physical Properties of Alkanes and Cycloalkanes
At room temperature and atmospheric pressure, simple saturated hydrocarbons and cycloalkanes are colorless gases, clear liquids, or white solids, depending on their molecular weight. To many people, they smell bad, although some of us think that these molecules have been the victims of a bad press and don’t smell bad at all. Cooking gas, which is mostly saturated hydrocarbons, has an odor that comes from a mercaptan (RSH) (Remember: R stands for a general alkyl group; p. 73), put in specifically so that escaping gas can be detected by smell.
Tables 2.4 and 2.6 show some physical properties of straight-chain and cyclic alkanes. Why do the boiling points increase as the number of carbons in the molecule increases? The boiling point is a measure of the ease of breaking up intermolecular attractive forces. The liquid phases of hydrocarbons are stabilized by a factor called van der Waals forces (after Johannes Diderik van der Waals, 1837–1923). These relatively weak forces arise because when two clouds of electrons approach each other, dipoles (molecules with two poles; p. 14) are induced as the clouds polarize in such a fashion as to stabilize each other by opposing plus and minus charges (Fig. 2.54).
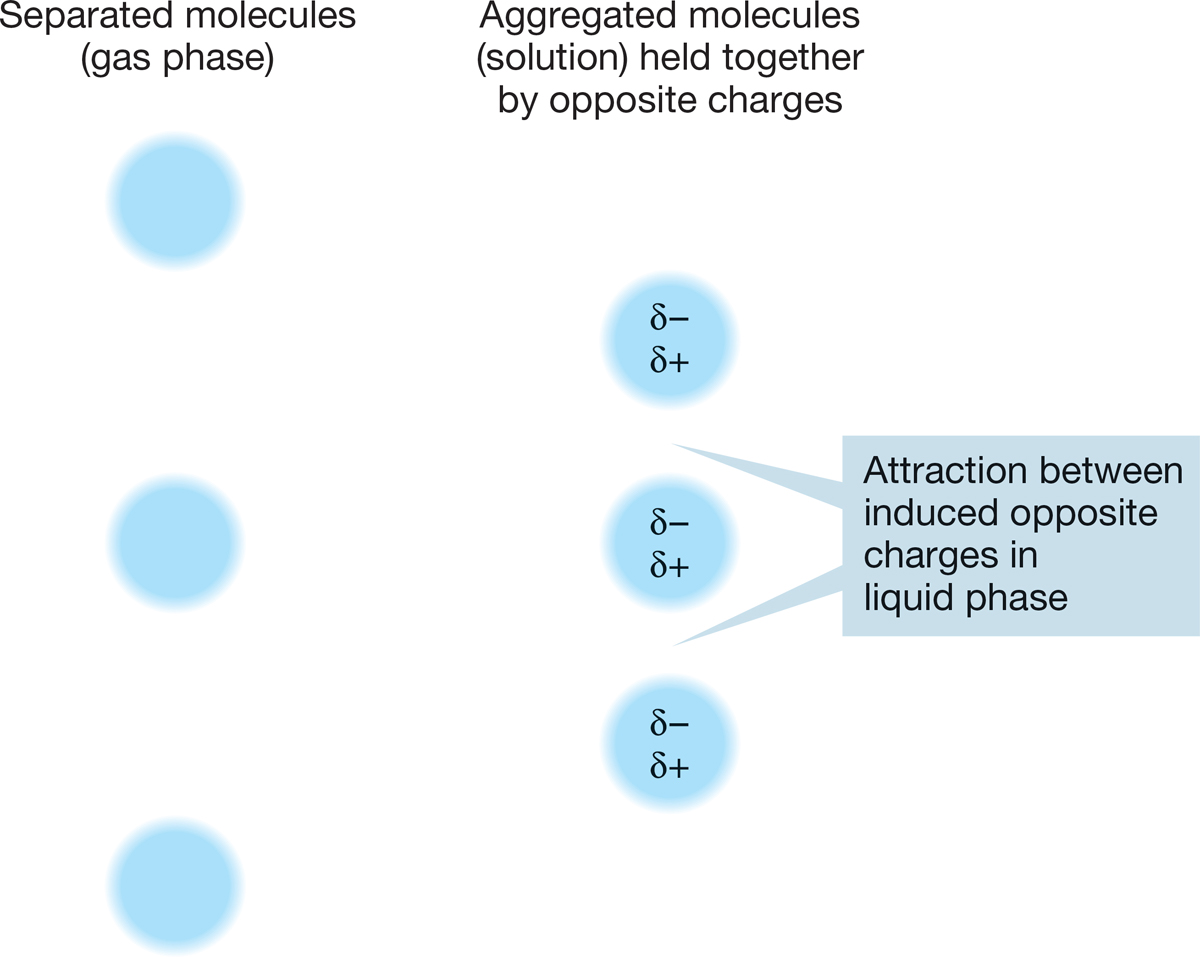
FIGURE 2.54 The stabilization of molecules through van der Waals forces.
Many alkanes have small dipoles to begin with, but they are very small and do not serve to hold the molecules together strongly. Thus, alkanes have relatively low boiling points. Other molecules are much more polar, and this polarity makes a big difference in boiling point. Polar molecules can associate quite strongly with each other by alignment of opposite charges. This association has the effect of increasing the boiling point.
The more extended a molecule is, the stronger its induced dipole can be. More compact, more spherical molecules have smaller induced dipoles and therefore lower boiling points. A classic example is the difference between pentane and neopentane (Fig. 2.55). The more spherical neopentane boils about 25 °C lower than the boiling point of the straight-chain isomer. Isopentane is less extended than pentane but more extended than neopentane, and its boiling point is right between the two, 30 °C.
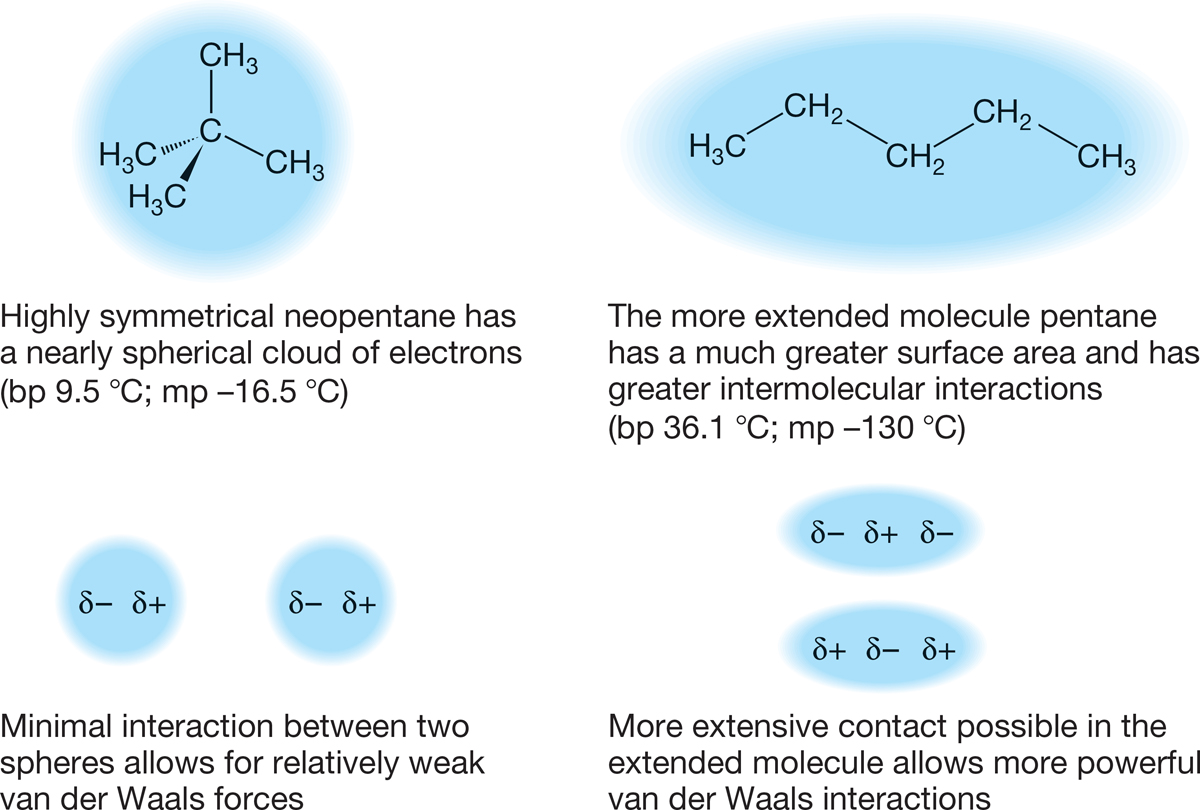
FIGURE 2.55 The more extended pentane boils at a higher temperature than that at which the more compact neopentane boils.
Symmetry is especially important in determining melting point because highly symmetric molecules pack well into crystal lattices. (Think of the computer game Tetris and how easy packing would be if every shape were a highly symmetrical square.) The better the packing of the lattice, the more energy it takes to break it up. So neopentane, for example, melts 113 °C higher than the melting point of pentane.