6.7 A Reaction of Alkyl Halides: Synthesis of Alkanes
Alkyl halides have all sorts of uses in the “real” practical world. They appear as anesthetics, insecticides, herbicides, the building blocks of polymers such as polyvinyl chloride (PVC), and myriad other products. As you read this paragraph, you may be thinking, “Sure, but haven’t there been serious problems with flame retardants such as brominated diphenyl ether and pesticides like chlordane, and weren’t refrigerants like chlorofluorocarbons eating up the ozone layer?” The answer is a resounding, Yes! There is no “free lunch,” as unintended consequences often appear when we try to solve problems with technology. The issues are complex, but it is clear that the chemists of today and of the future need to have the technical expertise to avoid these problems while retaining the benefits. So, study your “orgo”!
Alkyl halides are important in synthetic chemistry because they allow for the formation of carbon–hydrogen and carbon–carbon bonds. Central to these uses of alkyl and other halides is their easy conversion into organometallic reagents, molecules containing carbon–metal bonds. We look at only one limited use of these synthetically important compounds in this chapter, but many others will appear later. The reactions of the other substituted alkanes—the alcohols, the amines, and the ethers—will be topics of the upcoming chapters.
When an alkyl halide (fluorides are generally exceptions) is added to a cold mixture of magnesium or lithium metal and an ether solvent, the metal begins to dissolve in an exothermic reaction. In general, the ether solvent used for this reaction has the structure R―O―R. Diethyl ether or tetrahydrofuran (THF) are most often used. The end result of the reaction is either a Grignard reagent or an organolithium reagent (Fig. 6.54).
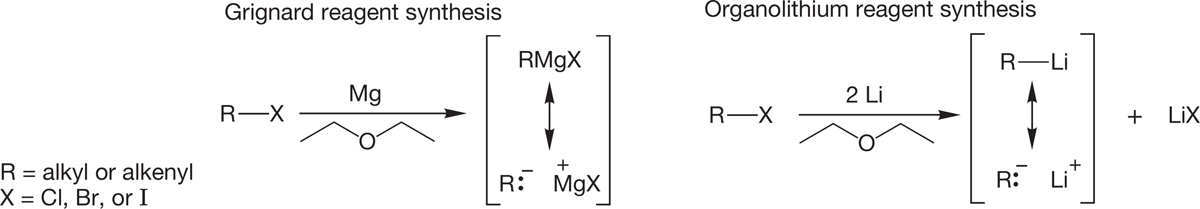
FIGURE 6.54 Formation of organometallic reagents.
Grignard reagents were discovered by P. A. Barbier (1848–1922), and their chemistry was worked out extensively by his student Victor Grignard (1871–1935), who received the 1912 Nobel Prize in Chemistry for his work in this area. Neither the formation of the Grignard reagent nor its structure is an easy subject. The simplest formulation is “RMgX” and should be amplified by the addition of the ionic resonance form shown in Figure 6.54. The Grignard reagent is also in equilibrium with a mixture of the magnesium halide and dialkylmagnesium compound. Moreover, the ether solvent is essential for its formation. Grignard reagents incorporate two molecules of solvent, conveniently left out in the traditional RMgX formulation (Fig. 6.55). Whatever the detailed structure, the result is a highly polar reagent that is a very strong Lewis base.
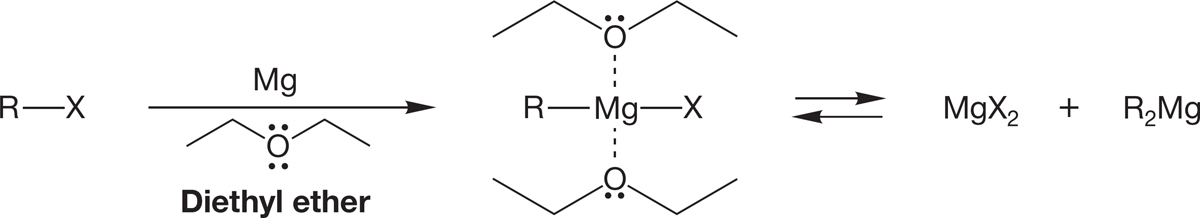
FIGURE 6.55 The Grignard reagent has a complex structure.
The mechanism of formation of the Grignard reagent involves radical-transfer reactions in which a transient alkyl radical is formed in the presence of a magnesium-centered radical (Fig. 6.56).

FIGURE 6.56 Formation of a Grignard reagent.
CONVENTION ALERT
Radicals
We discussed the methyl radical in Chapter 2 (p. 66). Radicals, sometimes called free radicals, are neutral species that have a single nonbonding electron and can undergo many reactions, as we will see in Chapter 12. Typical and important reactions of radicals include combining with other radicals to form bonds. The combination of two carbon-based radicals is especially important because it results in the formation of a carbon–carbon bond. The arrow formalism for radical reactions uses single-barbed arrows.
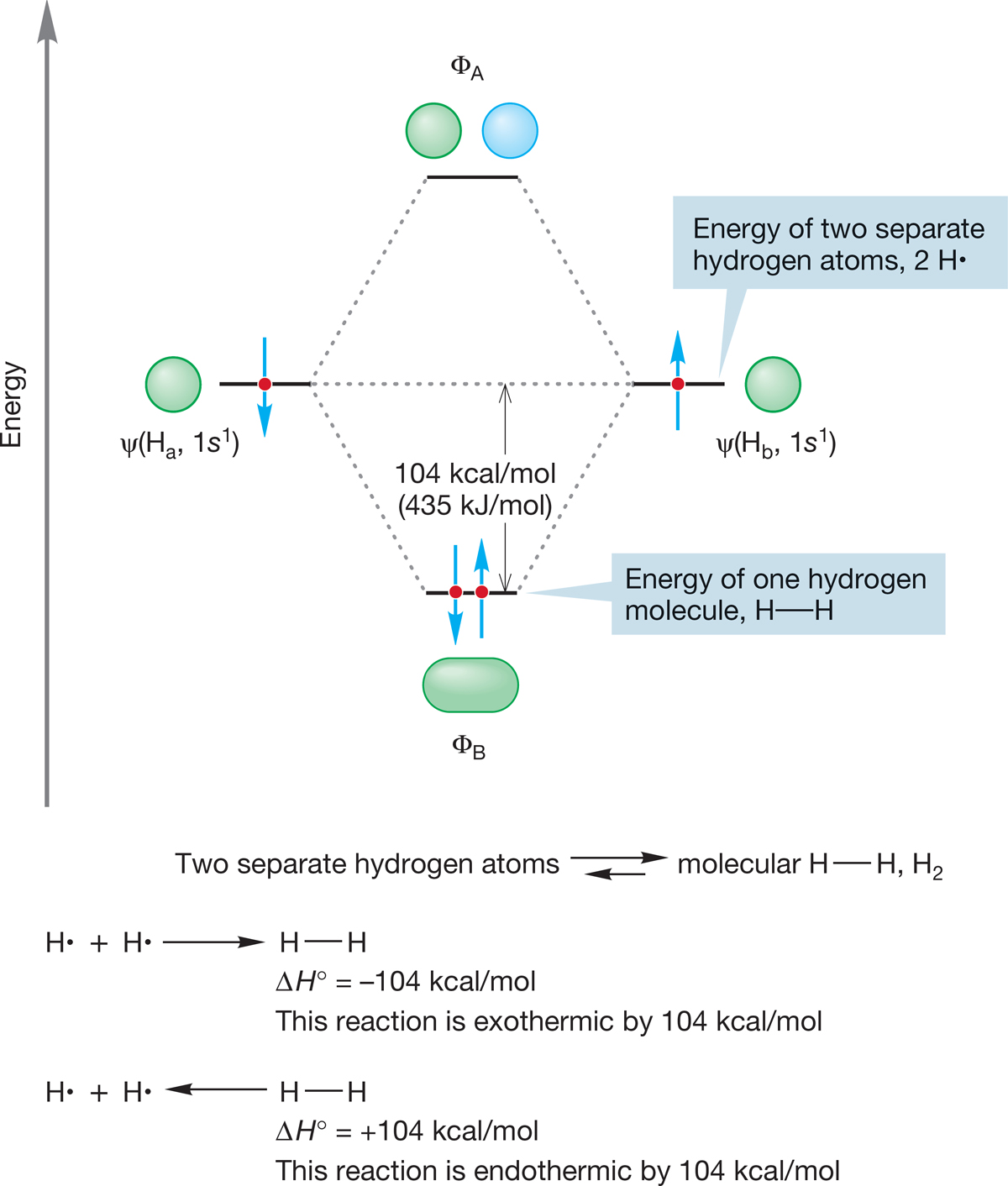
PROBLEM 6.18 Draw an interaction diagram to show the stabilization when two methyl radicals combine. How great is that stabilization? For an example of an interaction diagram, see Figure 1.44 (p. 38).
Coupling of R⋅ and ⋅MgX generates RMgX, and further radical reactions can equilibrate this species with the dialkylmagnesium. Most halides (except fluorides) can be made into Grignard reagents.

FIGURE 6.57 Organolithium reagents are not monomeric, but oligomeric. The exact value of n depends on the solvent and the structure of R.
Organolithium reagents are even more complex. Here the ether solvent is not essential, but the nature of the organolithium reagent does depend on the solvent. Organolithium reagents are known not to be monomeric; they form aggregates, the size of which depends on the nature of the solvent and the structure of the R group. As in RMgX, the representation of organolithium reagents as RLi, even with the addition of the ionic resonance form shown in Figure 6.54, is a convenient simplification (Fig. 6.57).
Both of these organometallic reagents are extraordinarily strong bases, and they must be carefully protected from moisture and oxygen. Chemists form them under a strictly dry and inert atmosphere. Although these reagents do not contain free carbanions, they act as if they did. They are sources of R:−. The very polar carbon–metal bond attacks all manner of Lewis and Brønsted acids. Water is more than strong enough to protonate a Grignard or organolithium reagent (Fig. 6.58).
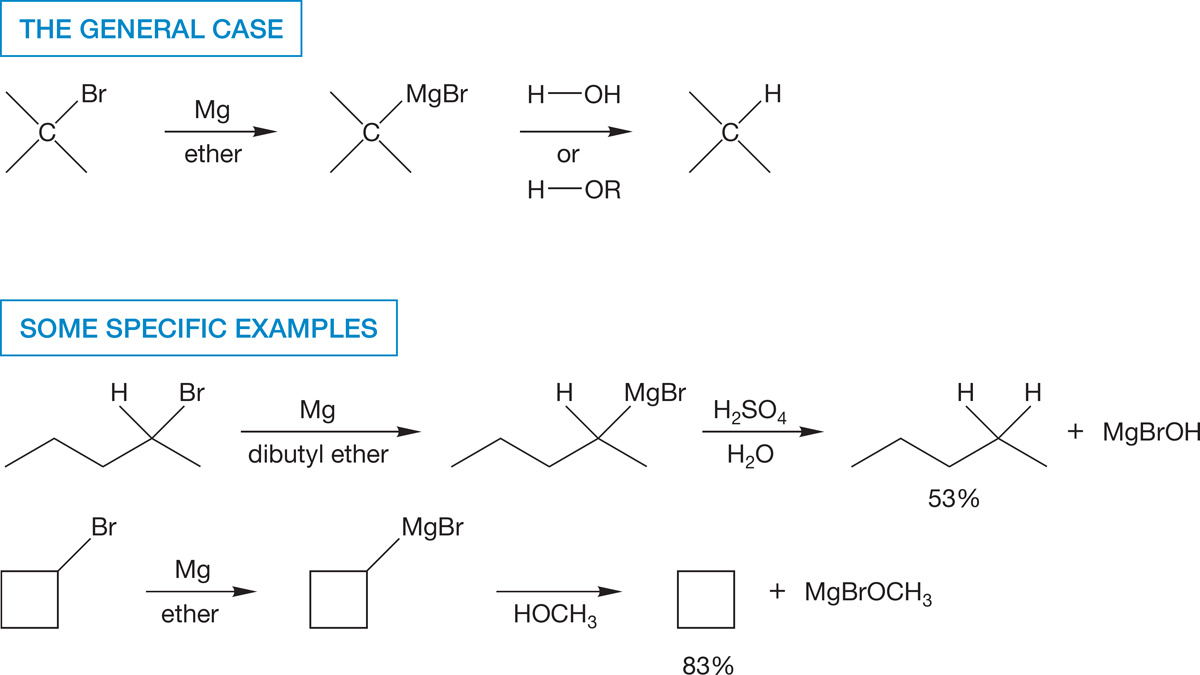
FIGURE 6.58 The powerfully basic organometallic reagents are easily protonated by water or an alcohol to give alkanes and metal hydroxides. Yields cited for specific examples are for R―Br to R―H.
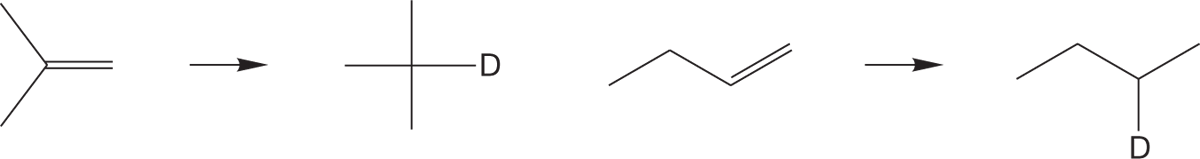
One of the end products of the reactions in Figure 6.58 is an alkane. This sequence constitutes a synthesis of alkanes from most halides (see Fig. 6.53). This reaction can often be put to good use in the construction of isotopically labeled reagents, because D2O can be used in place of H2O to produce a specifically deuteriolabeled alkane (Fig. 6.59).
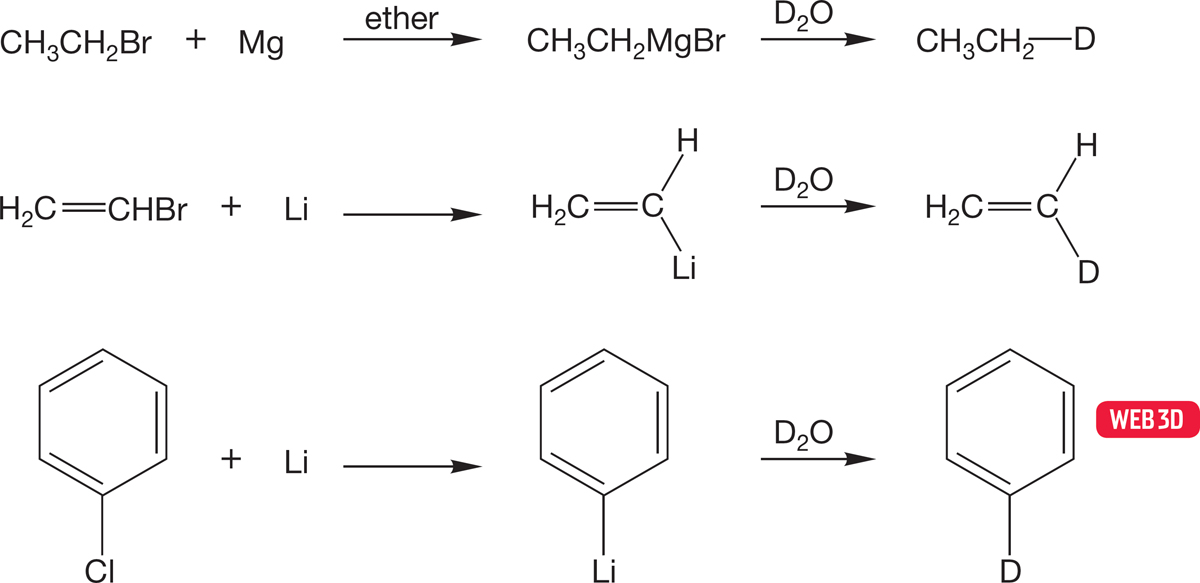
FIGURE 6.59 Treatment of an organometallic reagent with D2O gives deuteriolabeled molecules.
PROBLEM 6.19 Given inorganic reagents of your choice (including D2O), devise syntheses of the following molecules from the indicated starting materials: